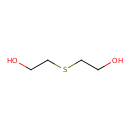
| 2-[(2-Hydroxyethyl)sulfanyl]ethan-1-ol | ChEBI |
| 3-Thiapentane-1,5-diol | ChEBI |
| beta,Beta'-dihydroxydiethyl sulfide | ChEBI |
| beta,Beta'-dihydroxyethyl sulfide | ChEBI |
| beta-Bis(hydroxyethyl) sulfide | ChEBI |
| beta-Hydroxyethyl sulfid | ChEBI |
| beta-Hydroxyethyl sulfide | ChEBI |
| beta-Thiodiglycol | ChEBI |
| Bis(2-hydroxyethyl) sulfide | ChEBI |
| Bis(2-hydroxyethyl) sulphide | ChEBI |
| Bis(2-hydroxyethyl) thioether | ChEBI |
| Bis(2-hydroxyethyl)sulfide | ChEBI |
| Bis(beta-hydroxyethyl) sulfide | ChEBI |
| Bis(beta-hydroxyethyl)sulfide | ChEBI |
| Bis(hydroxyethyl)sulfide | ChEBI |
| Di(2-hydroxyethyl) sulfide | ChEBI |
| Diethanol sulfide | ChEBI |
| Dihydroxyethyl sulfide | ChEBI |
| Thiodiethanol | ChEBI |
| Thiodiethylene glycol | ChEBI |
| Thiodiglycolum | ChEBI |
| Tiodiglicol | ChEBI |
| Tiodiglicolo | ChEBI |
| 2-[(2-Hydroxyethyl)sulphanyl]ethan-1-ol | Generator |
| b,Beta'-dihydroxydiethyl sulfide | Generator |
| b,Beta'-dihydroxydiethyl sulphide | Generator |
| beta,Beta'-dihydroxydiethyl sulphide | Generator |
| Β,beta'-dihydroxydiethyl sulfide | Generator |
| Β,beta'-dihydroxydiethyl sulphide | Generator |
| b,Beta'-dihydroxyethyl sulfide | Generator |
| b,Beta'-dihydroxyethyl sulphide | Generator |
| beta,Beta'-dihydroxyethyl sulphide | Generator |
| Β,beta'-dihydroxyethyl sulfide | Generator |
| Β,beta'-dihydroxyethyl sulphide | Generator |
| b-Bis(hydroxyethyl) sulfide | Generator |
| b-Bis(hydroxyethyl) sulphide | Generator |
| beta-Bis(hydroxyethyl) sulphide | Generator |
| Β-bis(hydroxyethyl) sulfide | Generator |
| Β-bis(hydroxyethyl) sulphide | Generator |
| b-Hydroxyethyl sulfid | Generator |
| b-Hydroxyethyl sulphid | Generator |
| beta-Hydroxyethyl sulphid | Generator |
| Β-hydroxyethyl sulfid | Generator |
| Β-hydroxyethyl sulphid | Generator |
| b-Hydroxyethyl sulfide | Generator |
| b-Hydroxyethyl sulphide | Generator |
| beta-Hydroxyethyl sulphide | Generator |
| Β-hydroxyethyl sulfide | Generator |
| Β-hydroxyethyl sulphide | Generator |
| b-Thiodiglycol | Generator |
| Β-thiodiglycol | Generator |
| Bis(2-hydroxyethyl)sulphide | Generator |
| Bis(b-hydroxyethyl) sulfide | Generator |
| Bis(b-hydroxyethyl) sulphide | Generator |
| Bis(beta-hydroxyethyl) sulphide | Generator |
| Bis(β-hydroxyethyl) sulfide | Generator |
| Bis(β-hydroxyethyl) sulphide | Generator |
| Bis(b-hydroxyethyl)sulfide | Generator |
| Bis(b-hydroxyethyl)sulphide | Generator |
| Bis(beta-hydroxyethyl)sulphide | Generator |
| Bis(β-hydroxyethyl)sulfide | Generator |
| Bis(β-hydroxyethyl)sulphide | Generator |
| Bis(hydroxyethyl)sulphide | Generator |
| Di(2-hydroxyethyl) sulphide | Generator |
| Diethanol sulphide | Generator |
| Dihydroxyethyl sulphide | Generator |
| 2,2'-Thiobis-ethanol | HMDB |
| 2,2'-Thiobisethanol | HMDB |
| 2,2'-Thiobis[ethanol] | HMDB |
| 2,2'-Thiodi-ethanol | HMDB |
| 2,2'-Thiodiethanol | HMDB |
| 2,2'-Thiodiglycol | HMDB |
| 2,2-Thiodiethanol | HMDB |
| Glyecine a | HMDB |
| Kromfax solvent | HMDB |
| Kromfax@ solvent | HMDB |
| Sulfide, bis(2-hydroxyethyl) | HMDB |
| Tedegyl | HMDB |
| 2,2'-Sulfobisethanol | HMDB |