| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:59:37 UTC |
|---|
| Update Date | 2016-11-09 01:13:56 UTC |
|---|
| Accession Number | CHEM009177 |
|---|
| Identification |
|---|
| Common Name | Glycidyl methacrylate |
|---|
| Class | Small Molecule |
|---|
| Description | An enoate ester obtained by formal condensation of the carboxy group of methacrylic acid with the hydroxy group of glycidol. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- OECD HPV Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
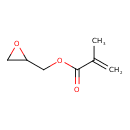
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3-Epoxypropanol methacrylate | ChEBI | | 2,3-Epoxypropyl methacrylate | ChEBI | | 2-((Methacryloxy)methyl)oxirane | ChEBI | | Glycidol methacrylate | ChEBI | | Glycidyl alpha-methylacrylate | ChEBI | | Methacrylic acid, 2,3-epoxypropyl ester | ChEBI | | 2,3-Epoxypropanol methacrylic acid | Generator | | 2,3-Epoxypropyl methacrylic acid | Generator | | Glycidol methacrylic acid | Generator | | Glycidyl a-methylacrylate | Generator | | Glycidyl a-methylacrylic acid | Generator | | Glycidyl alpha-methylacrylic acid | Generator | | Glycidyl α-methylacrylate | Generator | | Glycidyl α-methylacrylic acid | Generator | | Methacrylate, 2,3-epoxypropyl ester | Generator | | [(2R)-Oxiran-2-yl]methyl 2-methylprop-2-enoic acid | Generator | | Glycidyl methacrylic acid | HMDB | | Sta-lock | HMDB | | Monomethacrylate propylene oxide | HMDB | | Glycidyl methacrylate | HMDB |
|
|---|
| Chemical Formula | C7H10O3 |
|---|
| Average Molecular Mass | 142.154 g/mol |
|---|
| Monoisotopic Mass | 142.063 g/mol |
|---|
| CAS Registry Number | 106-91-2 |
|---|
| IUPAC Name | (oxiran-2-yl)methyl 2-methylprop-2-enoate |
|---|
| Traditional Name | glycidyl methacrylate |
|---|
| SMILES | CC(=C)C(=O)OCC1CO1 |
|---|
| InChI Identifier | InChI=1S/C7H10O3/c1-5(2)7(8)10-4-6-3-9-6/h6H,1,3-4H2,2H3 |
|---|
| InChI Key | VOZRXNHHFUQHIL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as enoate esters. These are an alpha,beta-unsaturated carboxylic ester of general formula R1C(R2)=C(R3)C(=O)OR4 (R4= organyl compound) in which the ester C=O function is conjugated to a C=C double bond at the alpha,beta position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acid derivatives |
|---|
| Direct Parent | Enoate esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Enoate ester
- Oxacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Ether
- Oxirane
- Dialkyl ether
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kf-9000000000-8e4e22f316bbdfcd18e2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-7900000000-83b88ea887d57bdee4b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9100000000-330983dc720f565052b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-ed153c546be4c48e91d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-9700000000-6853c6dfa0f94ed66ea1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9100000000-acd8d07b3331ce56de4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-9000000000-bd5e829879834d6082c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05mo-9100000000-f246b8050d1ded10941c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9000000000-71231048599a429f7eca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-1ec4a6c4fb43803b09a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0079-9100000000-3927fba63fbc4e0150e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-9000000000-b414029bb8dc77677115 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-8411530b003ba0051965 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0243756 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Glycidyl_methacrylate |
|---|
| Chemspider ID | 7549 |
|---|
| ChEBI ID | 132844 |
|---|
| PubChem Compound ID | 7837 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|