| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:59:05 UTC |
|---|
| Update Date | 2016-11-09 01:13:56 UTC |
|---|
| Accession Number | CHEM009147 |
|---|
| Identification |
|---|
| Common Name | Acetamide, N,N'-1,2-ethanediylbis[N-acetyl- |
|---|
| Class | Small Molecule |
|---|
| Description | Bleach activator used in food-contact paper and paperboard. TAED is an important component of detergents and bleaches. Its is an activator for "active oxygen" bleaching agents. Such active oxygen bleaching agents release hydrogen peroxide during the wash cycle. Such agents include sodium perborate, sodium percarbonate, sodium perphosphate, sodium persulfate, and urea peroxide. The released hydrogen peroxide is an inefficient bleach below 40 °C, except in the presence of activators such as TAED. Tetraacetylethylenediamine, commonly abbreviated TAED, is an organic compound with the formula (CH3C(O))2NCH2CH2N(C(O)CH3)2. This colourless compound is often dyed blue or green for use in laundry detergents, its most significant application. It is produced by acetylation of ethylenediamine. The activation process entails a reaction of the hydrogen peroxide with TAED to release peracetic acid, which is a fast-acting bleaching agent.:. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
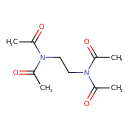
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Descarbamylnovobiocin | HMDB | | N,N'-1,2-ethanediylbis(N-acetyl-acetamide | HMDB | | N,N'-1,2-ethanediylbis[N-acetyl-acetamide | HMDB | | N,N'-1,2-ethanediylbis[N-acetylacetamide], 9ci | HMDB | | N,N'-ethylenebis(diacetamide) | HMDB | | N,N'-ethylenebis(diacetamide), 8ci | HMDB | | N,N'-ethylenebis(N-acetylacetamide) | HMDB | | N,N,N',n'-tetraacetylethylenediamine | HMDB | | N-Acetyl-N-[2-(diacetylamino)ethyl]acetamide | HMDB | | Tetracetylethylenediamine | HMDB | | TAED compound | MeSH |
|
|---|
| Chemical Formula | C10H16N2O4 |
|---|
| Average Molecular Mass | 228.245 g/mol |
|---|
| Monoisotopic Mass | 228.111 g/mol |
|---|
| CAS Registry Number | 10543-57-4 |
|---|
| IUPAC Name | N-acetyl-N-[2-(N-acetylacetamido)ethyl]acetamide |
|---|
| Traditional Name | tetraacetylethylenediamine |
|---|
| SMILES | CC(=O)N(CCN(C(C)=O)C(C)=O)C(C)=O |
|---|
| InChI Identifier | InChI=1S/C10H16N2O4/c1-7(13)11(8(2)14)5-6-12(9(3)15)10(4)16/h5-6H2,1-4H3 |
|---|
| InChI Key | BGRWYDHXPHLNKA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-substituted carboxylic acid imides. N-substituted carboxylic acid imides are compounds comprising an N-substituted carboxylic acid imide group, with the general structure R1N(C(R2)=O)C(R3)=O (R2,R3=H, alkyl, aryl; R1=Anything but H). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acid derivatives |
|---|
| Direct Parent | N-substituted carboxylic acid imides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carboxylic acid imide, n-substituted
- Acetamide
- Dicarboximide
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-7910000000-ba435445c9ed1e336841 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0490000000-50cba3ce1f4d7431aff3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1950000000-fc26b92e00340c6703de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000l-9500000000-9b78c4f755d7625b152f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0290000000-a5715dfa489c27d04bde | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004u-1940000000-ad50f46429638e81b165 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-4900000000-9df6cac8a1fbb023898d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-6950000000-34bd9877e15f12f88fbb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003i-9300000000-43dd4709082c14ca51dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9000000000-946aa1c67df13bf7fcca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004r-2890000000-50b333a05c701816edc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2900000000-346ef8a77073a37ec63e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-00eb271f345801081b12 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040573 |
|---|
| FooDB ID | FDB020351 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tetraacetylethylenediamine |
|---|
| Chemspider ID | 59725 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 66347 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|