| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:44:29 UTC |
|---|
| Update Date | 2016-11-09 01:13:47 UTC |
|---|
| Accession Number | CHEM008355 |
|---|
| Identification |
|---|
| Common Name | Metconazole II |
|---|
| Class | Small Molecule |
|---|
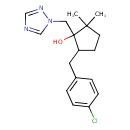
| Description | A member of the class of cyclopentanols carrying 1,2,4-triazol-1-ylmethyl and 4-chlorobenzyl and geminal dimethyl substituents at positions 1, 2 and 5 respectively. Used to control a range of fungal infections including alternaria, rusts, fusarium and septoria diseases. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-((4-Chlorophenyl)methyl)-2,2-dimethyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentan-1-ol | MeSH | | (1S,5R)-5-((4-Chlorophenyl)methyl)-2,2-dimethyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentan-1-ol | MeSH | | cis-Metconazole | MeSH |
|
|---|
| Chemical Formula | C17H22ClN3O |
|---|
| Average Molecular Mass | 319.830 g/mol |
|---|
| Monoisotopic Mass | 319.145 g/mol |
|---|
| CAS Registry Number | 999021-03-3 |
|---|
| IUPAC Name | 5-[(4-chlorophenyl)methyl]-2,2-dimethyl-1-[(1H-1,2,4-triazol-1-yl)methyl]cyclopentan-1-ol |
|---|
| Traditional Name | metconazole |
|---|
| SMILES | CC1(C)CCC(CC2=CC=C(Cl)C=C2)C1(O)CN1C=NC=N1 |
|---|
| InChI Identifier | InChI=1S/C17H22ClN3O/c1-16(2)8-7-14(9-13-3-5-15(18)6-4-13)17(16,22)10-21-12-19-11-20-21/h3-6,11-12,14,22H,7-10H2,1-2H3 |
|---|
| InChI Key | XWPZUHJBOLQNMN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as iridoids and derivatives. These are monoterpenes containing a skeleton structurally characterized by the presence of a cylopentane fused to a pyran ( forming a 4,7-dimethylcyclopenta[c]pyran), or a derivative where the pentane moiety is open. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Iridoids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aromatic monoterpenoid
- 11-noriridane monoterpenoid
- Monocyclic monoterpenoid
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- Cyclopentanol
- Benzenoid
- 1,2,4-triazole
- Tertiary alcohol
- Azole
- Cyclic alcohol
- Heteroaromatic compound
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Organohalogen compound
- Organochloride
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0019000000-f36e8edc69ebb59d2ef9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-0090000000-46393d8763672bc5cfe7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9200000000-f4e566cd0cb206740134 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-9003000000-019d5ccd41a9ab9e186d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9012000000-7264989e7366b6de30b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-839ef7865d2581933604 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 81773 |
|---|
| PubChem Compound ID | 86210 |
|---|
| Kegg Compound ID | C18476 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|