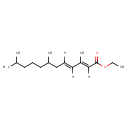
| Synonyms | | Value | Source |
|---|
| (2E,4E)-3,7,11-Trimethyldodeca-2,4-dienoic acid, ethyl ester | ChEBI | | (2E,4E)-Hydroprene | ChEBI | | (e,e)-3,7,11-Trimethyl-2,4-dodecadienoic acid, ethyl ester | ChEBI | | (RS)-Hydroprene | ChEBI | | Ethyl (2E,4E)-3,7,11-trimethyl-2,4-dodecadienoate | ChEBI | | Ethyl (e,e)-(+-)-3,7,11-trimethyl-2,4-dodecadienoate | ChEBI | | Ethyl (e,e)-3,7,11-trimethyl-2,4-dodecadienoate | ChEBI | | Ethyl 3,7,11-trimethyl-trans-2,trans-4-dodecadienoate | ChEBI | | Gencor | ChEBI | | ZR 512 | ChEBI | | (2E,4E)-3,7,11-Trimethyldodeca-2,4-dienoate, ethyl ester | Generator | | (e,e)-3,7,11-Trimethyl-2,4-dodecadienoate, ethyl ester | Generator | | Ethyl (2E,4E)-3,7,11-trimethyl-2,4-dodecadienoic acid | Generator | | Ethyl (e,e)-(+-)-3,7,11-trimethyl-2,4-dodecadienoic acid | Generator | | Ethyl (e,e)-3,7,11-trimethyl-2,4-dodecadienoic acid | Generator | | Ethyl 3,7,11-trimethyl-trans-2,trans-4-dodecadienoic acid | Generator | | Ethyl (2E,4E)-3,7,11-trimethyldodeca-2,4-dienoic acid | Generator | | Hydroprene | MeSH | | Ethyl-3,7,11-trimethyl-2,4-dodecadienoate, ((e,e)-(+-))-isomer | MeSH | | Ethyl-3,7,11-trimethyl-2,4-dodecadienoate, (e,Z)-isomer | MeSH | | Ethyl-3,7,11-trimethyl-2,4-dodecadienoate, (R-(e,e))-isomer | MeSH | | Ethyl-3,7,11-trimethyl-2,4-dodecadienoate, ((Z,Z)-(+-))-isomer | MeSH | | Ethyl-3,7,11-trimethyl-2,4-dodecadienoate, (S-(e,e))-isomer | MeSH | | Ethyl-3,7,11-trimethyl-2,4-dodecadienoate, (Z,e)-isomer | MeSH | | Altozar | MeSH | | Ethyl-3,7,11-trimethyl-2,4-dodecadienoate | MeSH | | Ethyl-3,7,11-trimethyl-2,4-dodecadienoate, (Z,Z)-isomer | MeSH |
|
|---|