| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:39:35 UTC |
|---|
| Update Date | 2016-10-28 10:04:41 UTC |
|---|
| Accession Number | CHEM008151 |
|---|
| Identification |
|---|
| Common Name | Phthalimide |
|---|
| Class | Small Molecule |
|---|
| Description | A dicarboximide that is 2,3-dihydro-1H-isoindole substituted by oxo groups at positions 1 and 3. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- My Exposome Chemicals
- OECD HPV Chemicals
- STOFF IDENT Compounds
- Suspected Compounds
- Suspected Compounds – Schymanski Project
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
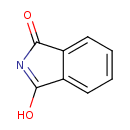
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,3-Isoindolinedione | ChEBI | | 1H-Isoindole-1,3(2H)-dione | ChEBI | | 2-Diazoindan-1,3-dione | ChEBI | | O-Phthalic imide | ChEBI | | Potassium phthalimide | MeSH | | Phthalimide calcium (2:1) salt | MeSH | | Phthalimide potassium salt | MeSH | | Phthalimide | MeSH |
|
|---|
| Chemical Formula | C8H5NO2 |
|---|
| Average Molecular Mass | 147.133 g/mol |
|---|
| Monoisotopic Mass | 147.032 g/mol |
|---|
| CAS Registry Number | 85-41-6 |
|---|
| IUPAC Name | 3-hydroxy-1H-isoindol-1-one |
|---|
| Traditional Name | phthalimide |
|---|
| SMILES | OC1=NC(=O)C2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C8H5NO2/c10-7-5-3-1-2-4-6(5)8(11)9-7/h1-4H,(H,9,10,11) |
|---|
| InChI Key | XKJCHHZQLQNZHY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phthalimides. These are aromatic heterocyclic compounds containing a 1,3-dioxoisoindoline moiety. They are imide derivatives of phthalic anhydrides. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoindoles and derivatives |
|---|
| Sub Class | Isoindolines |
|---|
| Direct Parent | Phthalimides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phthalimide
- Isoindole
- Benzenoid
- Carboxylic acid imide, n-unsubstituted
- Carboxylic acid imide
- Azacycle
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-2900000000-87ae2d9bb5a7af9927f7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-001i-0900000000-441496a21fe7006c2b36 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0002-0900000000-4ce0101e8df3884967de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-2bad38f61a6230a21687 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-27417e3fbf264981c393 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6t-6900000000-f4c7d426c06195f49d1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-57e71b2c2b03fb207747 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-942c963788c348ada15a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-ee566042866f9725b6de | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| | Status | Value | Unit | Sample Location | Reference |
|---|
|
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0256502 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Phthalimide |
|---|
| Chemspider ID | 6550 |
|---|
| ChEBI ID | 38817 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|