| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:39:28 UTC |
|---|
| Update Date | 2016-11-09 01:13:44 UTC |
|---|
| Accession Number | CHEM008147 |
|---|
| Identification |
|---|
| Common Name | 2,4,6-Trichloroanisole |
|---|
| Class | Small Molecule |
|---|
| Description | A monomethoxybenzene that is 1,3,5-trichlorobenzene in which one of the hydrogens is replaced by a methoxy group. |
|---|
| Contaminant Sources | - FooDB Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
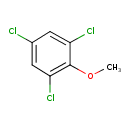
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 2,4,6-trichlorophenyl ether | ChEBI | | Tyrene | ChEBI | | 1,3,5-Trichloro-2-methoxy-benzene | HMDB | | 1,3,5-Trichloro-2-methoxybenzene, 9ci | HMDB | | 2,4,6-Trichloro-1-methoxybenzene | HMDB | | 2,4,6-Trichloro-anisole | HMDB | | 2,4,6-Trichloroanisole | HMDB | | 2,4.6-Trichloroanisole | HMDB | | Anisole, 2,4,6-trichloro- (8ci) | HMDB | | Benzene, 1,3,5-trichloro-2-methoxy- (9ci) | HMDB | | Trichloroanisole | HMDB | | 1,3,5-Trichloro-2-methoxybenzene | ChEBI |
|
|---|
| Chemical Formula | C7H5Cl3O |
|---|
| Average Molecular Mass | 211.473 g/mol |
|---|
| Monoisotopic Mass | 209.941 g/mol |
|---|
| CAS Registry Number | 87-40-1 |
|---|
| IUPAC Name | 1,3,5-trichloro-2-methoxybenzene |
|---|
| Traditional Name | tyrene |
|---|
| SMILES | COC1=C(Cl)C=C(Cl)C=C1Cl |
|---|
| InChI Identifier | InChI=1S/C7H5Cl3O/c1-11-7-5(9)2-4(8)3-6(7)10/h2-3H,1H3 |
|---|
| InChI Key | WCVOGSZTONGSQY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anisoles. These are organic compounds containing a methoxybenzene or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenol ethers |
|---|
| Sub Class | Anisoles |
|---|
| Direct Parent | Anisoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenoxy compound
- Anisole
- Methoxybenzene
- Alkyl aryl ether
- Chlorobenzene
- Halobenzene
- Monocyclic benzene moiety
- Aryl halide
- Aryl chloride
- Ether
- Organohalogen compound
- Organic oxygen compound
- Organochloride
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08mi-0960000000-de8ef54c1bb1a0ac1a78 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-f529af1a01e3b19a2322 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0090000000-543a39e57d4773a9995e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01q9-0940000000-d2c67a78d4de81a333fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-7e3c76ed1a9950c7fc8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-7e3c76ed1a9950c7fc8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0490000000-183c8bade61db0ff3383 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-bb489de3ef7ca6fbf745 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-bb489de3ef7ca6fbf745 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-c2fa753da65a4bac80a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-effa10fd233819efb3f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0090000000-effa10fd233819efb3f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0229-0930000000-07798209e5ae4e2a7449 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029643 |
|---|
| FooDB ID | FDB000814 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00017818 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 6620 |
|---|
| ChEBI ID | 19333 |
|---|
| PubChem Compound ID | 6884 |
|---|
| Kegg Compound ID | C11510 |
|---|
| YMDB ID | YMDB01597 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|