| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:37:15 UTC |
|---|
| Update Date | 2016-11-09 01:13:43 UTC |
|---|
| Accession Number | CHEM008071 |
|---|
| Identification |
|---|
| Common Name | Phenthoate |
|---|
| Class | Small Molecule |
|---|
| Description | An organic thiophosphate that is ethyl mandelate in which the hydroxy group has been replaced by a (dimethoxyphosphorothioyl)sulfanediyl group. |
|---|
| Contaminant Sources | - My Exposome Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
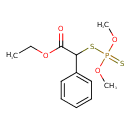
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dimephenthioate | ChEBI | | Ethyl alpha-((dimethoxyphosphenothioyl)thio)benzeneacetate | ChEBI | | Fenthoate | ChEBI | | O,O-Dimethyl S-alpha-ethoxycarbonylbenzyl phosphorodithioate | ChEBI | | S-[alpha-(Ethoxycarbonyl)benzyl] O,O-dimethyl phosphorodithioate | ChEBI | | Dimephenthioic acid | Generator | | Ethyl a-((dimethoxyphosphenothioyl)thio)benzeneacetate | Generator | | Ethyl a-((dimethoxyphosphenothioyl)thio)benzeneacetic acid | Generator | | Ethyl alpha-((dimethoxyphosphenothioyl)thio)benzeneacetic acid | Generator | | Ethyl α-((dimethoxyphosphenothioyl)thio)benzeneacetate | Generator | | Ethyl α-((dimethoxyphosphenothioyl)thio)benzeneacetic acid | Generator | | Fenthoic acid | Generator | | O,O-Dimethyl S-a-ethoxycarbonylbenzyl phosphorodithioate | Generator | | O,O-Dimethyl S-a-ethoxycarbonylbenzyl phosphorodithioic acid | Generator | | O,O-Dimethyl S-alpha-ethoxycarbonylbenzyl phosphorodithioic acid | Generator | | O,O-Dimethyl S-α-ethoxycarbonylbenzyl phosphorodithioate | Generator | | O,O-Dimethyl S-α-ethoxycarbonylbenzyl phosphorodithioic acid | Generator | | S-[a-(Ethoxycarbonyl)benzyl] O,O-dimethyl phosphorodithioate | Generator | | S-[a-(Ethoxycarbonyl)benzyl] O,O-dimethyl phosphorodithioic acid | Generator | | S-[alpha-(Ethoxycarbonyl)benzyl] O,O-dimethyl phosphorodithioic acid | Generator | | S-[Α-(ethoxycarbonyl)benzyl] O,O-dimethyl phosphorodithioate | Generator | | S-[Α-(ethoxycarbonyl)benzyl] O,O-dimethyl phosphorodithioic acid | Generator | | Phenthoic acid | Generator | | Ethyl 2-dimethoxyphosphinothioylsulfanyl-2-phenylacetic acid | Generator | | Ethyl 2-dimethoxyphosphinothioylsulphanyl-2-phenylacetate | Generator | | Ethyl 2-dimethoxyphosphinothioylsulphanyl-2-phenylacetic acid | Generator | | Elsan | MeSH | | Phenthoate | MeSH | | Phenthoate, (+)-isomer | MeSH | | Phenthoate, (+-)-isomer | MeSH | | Phendal | MeSH | | Cidial | MeSH | | Cidiale | MeSH | | Phenthoate, (-)-isomer | MeSH | | O,O-Dimethyl S-alpha-ethoxycarbonylbenzylphosphorodithioate | MeSH |
|
|---|
| Chemical Formula | C12H17O4PS2 |
|---|
| Average Molecular Mass | 320.360 g/mol |
|---|
| Monoisotopic Mass | 320.031 g/mol |
|---|
| CAS Registry Number | 254642 |
|---|
| IUPAC Name | ethyl 2-{[dimethoxy(sulfanylidene)-λ⁵-phosphanyl]sulfanyl}-2-phenylacetate |
|---|
| Traditional Name | elsan |
|---|
| SMILES | CCOC(=O)C(SP(=S)(OC)OC)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C12H17O4PS2/c1-4-16-12(13)11(10-8-6-5-7-9-10)19-17(18,14-2)15-3/h5-9,11H,4H2,1-3H3 |
|---|
| InChI Key | XAMUDJHXFNRLCY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzene and substituted derivatives. These are aromatic compounds containing one monocyclic ring system consisting of benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzene and substituted derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocyclic benzene moiety
- Dithiophosphate o-ester
- Dithiophosphate s-ester
- Organic dithiophosphate
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organothiophosphorus compound
- Sulfenyl compound
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Organosulfur compound
- Carbonyl group
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-1290000000-c5f01f7db279529e5827 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0072-0391000000-b77424b4c59b6ed5cd10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0190000000-0aa79cd06ff7e619a336 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-1930000000-fdd231bbaf6703ea27b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00rj-1193000000-61c083c9e8fdb76ae727 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-2191000000-daac06484737039b15da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0900-1390000000-fcd4b4f2c7875041d051 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0256420 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Phenthoate |
|---|
| Chemspider ID | 16492 |
|---|
| ChEBI ID | 34917 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C14429 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|