| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:35:55 UTC |
|---|
| Update Date | 2016-11-09 01:13:42 UTC |
|---|
| Accession Number | CHEM008022 |
|---|
| Identification |
|---|
| Common Name | Dinoseb acetate |
|---|
| Class | Small Molecule |
|---|
| Description | Postemergence herbicid |
|---|
| Contaminant Sources | - FooDB Chemicals
- My Exposome Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
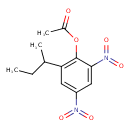
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dinoseb acetic acid | Generator | | 2,4-Dinitro-6-S-butylfenylester kyseliny octove | HMDB | | 2,4-Dinitro-6-sek.butyl-phenylacetat | HMDB | | 2-(1-Methylpropyl)-4,6-dinitrophenyl acetate | HMDB | | 2-(2-Hydroxy-3,5-dinitrophenyl)butane acetate | HMDB | | 2-Sec-butyl-4,6-dinitrophenol acetate (ester) | HMDB | | 2-Sec-butyl-4,6-dinitrophenyl acetate | HMDB | | 2-Sec-butyl-4,6-dinitrophenylacetate | HMDB | | 2-Sek.Butyl-4,6-dinitrofenylester kyseliny octove | HMDB | | 4,6-Dinitro-2-S-butylphenyl acetate | HMDB | | 6-Sec-butyl-2,4-dinitrophenylacetate | HMDB | | Acetic acid, (2,4-dinitro-6-S-butylphenyl) ester | HMDB | | Acetic acid, (4,6-dinitro-2-S-butylphenyl) ester | HMDB | | Acetic acid, 2-(sec-butyl)-4,6-dinitrophenyl ester | HMDB | | beta-(2-Hydroxy-3,5-dinitrophenyl)butane acetate | HMDB | | Dinoseb acetate, ansi, bsi, iso, wssa | HMDB | | Dinoseb-acetate | HMDB | | Dinosebe acetate | HMDB | | DNBPA, jmaf | HMDB | | O-Acetyl-2-sec-butyl-4,6-dinitrophenol | HMDB | | Phenol, 2-(1-methylpropyl)-4,6-dinitro-, acetate | HMDB | | Phenol, 2-(1-methylpropyl)-4,6-dinitro-, acetate (ester) | HMDB | | Phenol, 2-sec-butyl-4,6-dinitro-, acetate | HMDB | | Phenol, 2-sec-butyl-4,6-dinitro-, acetate (ester) | HMDB | | Phenol, 2-sec-butyl-4,6-dinitro-, acetate (ester) (8ci) | HMDB | | Aretit | HMDB |
|
|---|
| Chemical Formula | C12H14N2O6 |
|---|
| Average Molecular Mass | 282.249 g/mol |
|---|
| Monoisotopic Mass | 282.085 g/mol |
|---|
| CAS Registry Number | 2813-95-8 |
|---|
| IUPAC Name | 2-(butan-2-yl)-4,6-dinitrophenyl acetate |
|---|
| Traditional Name | dinoseb acetate |
|---|
| SMILES | CCC(C)C1=C(OC(C)=O)C(=CC(=C1)N(=O)=O)N(=O)=O |
|---|
| InChI Identifier | InChI=1S/C12H14N2O6/c1-4-7(2)10-5-9(13(16)17)6-11(14(18)19)12(10)20-8(3)15/h5-7H,4H2,1-3H3 |
|---|
| InChI Key | RDJTWDKSYLLHRW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenol esters. These are aromatic compounds containing a benzene ring substituted by a hydroxyl group and an ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenol esters |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenol esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenol ester
- Nitrobenzene
- Phenylpropane
- Phenoxy compound
- Nitroaromatic compound
- Monocyclic benzene moiety
- Carboxylic acid ester
- C-nitro compound
- Organic nitro compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic oxoazanium
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic 1,3-dipolar compound
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ox-3190000000-b098545c3ff4adc005b2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-a228d2dad11ecafa9cdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0090000000-1d3386e561f426b64a17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9060000000-ed3498127e8573322d17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-5951be86c97941995a38 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1090000000-05f818ceadd5e6e0adbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9040000000-9c748b9a0fe902933d08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-4750e3a05dba7e0bc074 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1090000000-53ae5e236e6d9f6d9b98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000b-9520000000-3c31f439cb4b56e11849 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-30a104bc02aa11a3034d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0089-0090000000-d439c5d1a5828199815e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-4920000000-f9836c8e31cb3023a885 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032560 |
|---|
| FooDB ID | FDB010492 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 16798 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 17776 |
|---|
| Kegg Compound ID | C19119 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|