| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:35:18 UTC |
|---|
| Update Date | 2016-11-09 01:13:42 UTC |
|---|
| Accession Number | CHEM008002 |
|---|
| Identification |
|---|
| Common Name | Iprobenfos |
|---|
| Class | Small Molecule |
|---|
| Description | An organic thiophosphate that is the S-benzyl O,O-diisopropyl ester of phosphorothioic acid. Used as a rice fungicide to control leaf and ear blast, stem rot and sheath blight. |
|---|
| Contaminant Sources | - FooDB Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
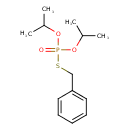
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Kitazin p | ChEBI | | O,O-Bis(1-methylethyl) S-(phenylmethyl)phosphorothioate | ChEBI | | O,O-Diisopropyl S-benzyl phosphorothioate | ChEBI | | O,O-DIIsopropyl S-benzyl thiophosphate | ChEBI | | Phosphorothioic acid, S-benzyl O,O-diisopropyl ester | ChEBI | | S-Benzyl diisopropyl phosphorothioate | ChEBI | | S-Benzyl O,O-diisopropyl phosphorothioate | ChEBI | | S-Benzyl O,O-diisopropyl thiophosphate | ChEBI | | IBP | Kegg | | O,O-Bis(1-methylethyl) S-(phenylmethyl)phosphorothioic acid | Generator | | O,O-Diisopropyl S-benzyl phosphorothioic acid | Generator | | O,O-DIIsopropyl S-benzyl thiophosphoric acid | Generator | | Phosphorothioate, S-benzyl O,O-diisopropyl ester | Generator | | S-Benzyl diisopropyl phosphorothioic acid | Generator | | S-Benzyl O,O-diisopropyl phosphorothioic acid | Generator | | S-Benzyl O,O-diisopropyl thiophosphoric acid | Generator | | Iprofenfos | HMDB | | Kitazin L | HMDB | | O,O-Bis(1-methylethyl) S-phenylmethyl phosphorothioate | HMDB | | O,O-Bis(1-methylethyl) S-phenylmethyl phosphorothioate, 9ci | HMDB | | O,O-DIIsopropyl S-benzyl phosphorothiolate | HMDB | | O,O-DIIsopropyl-S-benzylester kyseliny thiofosforecne | HMDB | | O,O-DIIsopropyl-S-benzylthiophosphate | HMDB | | Ricid II | HMDB | | Ricid p | HMDB | | S-Benzyl diisopropylphosphorothiolate | HMDB | | S-Benzyl O,O-diisopropyl phosphorothioate, 8ci | HMDB | | S-Benzyl-O,o'-diisopropylphosphorothiolate | HMDB |
|
|---|
| Chemical Formula | C13H21O3PS |
|---|
| Average Molecular Mass | 288.343 g/mol |
|---|
| Monoisotopic Mass | 288.095 g/mol |
|---|
| CAS Registry Number | 26087-47-8 |
|---|
| IUPAC Name | bis(propan-2-yl) (benzylsulfanyl)phosphonate |
|---|
| Traditional Name | iprobenfos |
|---|
| SMILES | CC(C)OP(=O)(OC(C)C)SCC1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C13H21O3PS/c1-11(2)15-17(14,16-12(3)4)18-10-13-8-6-5-7-9-13/h5-9,11-12H,10H2,1-4H3 |
|---|
| InChI Key | FCOAHACKGGIURQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzene and substituted derivatives. These are aromatic compounds containing one monocyclic ring system consisting of benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzene and substituted derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocyclic benzene moiety
- Sulfenyl compound
- Organothiophosphorus compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9240000000-05cdde724a5f17e5a1ca | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0kbb-2690000000-c8fecad2b510946b3043 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1190000000-196c1f793390eaffef38 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-7bf887779624c6e2a5e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000b-0690000000-56feb99d89cdf2b3ee7c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ea-1960000000-6571030797dbd11c5e29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fsr-0940000000-09868fec081088f35cc7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0930000000-5c24cae99992dd6ac5bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0930000000-e93d841bcc8e0c24c5c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-0900000000-187ebd551a11fb3c6543 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4j-0190000000-c0dcb172390b4ba25eea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9610000000-0325cb83fcdfa8f0b2a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9500000000-913aa31ef3c835eefc29 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031768 |
|---|
| FooDB ID | FDB008441 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30753 |
|---|
| ChEBI ID | 79737 |
|---|
| PubChem Compound ID | 33294 |
|---|
| Kegg Compound ID | C15230 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|