| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:34:58 UTC |
|---|
| Update Date | 2016-11-09 01:13:42 UTC |
|---|
| Accession Number | CHEM007989 |
|---|
| Identification |
|---|
| Common Name | Pyroquilon |
|---|
| Class | Small Molecule |
|---|
| Description | A pyrroloquinoline that is 1,2,5,6-tetrahydro-4H-pyrrolo[3,2,1-ij]quinoline in which the hydrogens at position 4 are replaced by an oxo group. A fungicide used to control rice blast, it is not approved wof use within the European Union. |
|---|
| Contaminant Sources | - FooDB Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,5,6-Tetrahydropyrrolo[3,2,1-ij]quinolin-4-one | ChEBI | | Coratop | ChEBI | | Fongoren | ChEBI | | Fongorene | ChEBI | | Lilolidone | ChEBI | | Pyroquilone | ChEBI | | 1,2,5,6-tetrahydro-4H-pyrrolo(3,2,1-Ij)quinolin-4-one | HMDB | | 1,2,5,6-tetrahydro-4H-pyrrolo(3,2,1-Ij)quinolin-4-one (9ci) | HMDB | | 1,2,5,6-tetrahydropyrrolo(3,2,1-Ij)quinolin-4-one | HMDB | | 4-Oxolilidine | HMDB | | PYQ | HMDB | | Pyroquilon | HMDB | | Pyroquilon, bsi, iso | HMDB |
|
|---|
| Chemical Formula | C11H11NO |
|---|
| Average Molecular Mass | 173.211 g/mol |
|---|
| Monoisotopic Mass | 173.084 g/mol |
|---|
| CAS Registry Number | 57369-32-1 |
|---|
| IUPAC Name | 1-azatricyclo[6.3.1.0⁴,¹²]dodeca-4(12),5,7-trien-11-one |
|---|
| Traditional Name | pyroquilon |
|---|
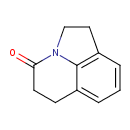
| SMILES | O=C1CCC2=CC=CC3=C2N1CC3 |
|---|
| InChI Identifier | InChI=1S/C11H11NO/c13-10-5-4-8-2-1-3-9-6-7-12(10)11(8)9/h1-3H,4-7H2 |
|---|
| InChI Key | XRJLAOUDSILTFT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroquinolones. Hydroquinolones are compounds containing a hydrogenated quinoline bearing a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Quinolones and derivatives |
|---|
| Direct Parent | Hydroquinolones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydroquinolone
- Tetrahydroquinoline
- Indole or derivatives
- Benzenoid
- Tertiary carboxylic acid amide
- Carboxamide group
- Lactam
- Carboxylic acid derivative
- Azacycle
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-008a-0900000000-ffe4f8f6416b7f66037b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-d97975a5b1dd3dc54b00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-dc8b09bb91da65b7dbe6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-0900000000-9e49a756e944af4820c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-d009fa59ccfee463f488 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-07e9b055551e5f2fdfc6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-2900000000-94c145da43ec5d6eff51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-3473e1d34af1c83c8904 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-bd044b74560d63d23b23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06dj-0900000000-fff91be4fd40fa7f2f82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-4c1ad417189eea157a87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-4c1ad417189eea157a87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-0900000000-481b117a0ebc4aa207b7 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB02756 |
|---|
| HMDB ID | HMDB0037113 |
|---|
| FooDB ID | FDB016106 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | PYQ |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 82768 |
|---|
| ChEBI ID | 45141 |
|---|
| PubChem Compound ID | 91665 |
|---|
| Kegg Compound ID | C18487 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|