| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:30:39 UTC |
|---|
| Update Date | 2016-11-09 01:13:40 UTC |
|---|
| Accession Number | CHEM007824 |
|---|
| Identification |
|---|
| Common Name | VERBENOL |
|---|
| Class | Small Molecule |
|---|
| Description | Verbenol is found in hyssop. Verbenol is a flavouring ingredien |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
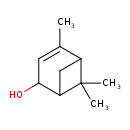
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Verbenol | HMDB | | (e)-Verbenol | HMDB | | (S)-cis-Verbenol | HMDB | | 2-Pinen-4-ol (8ci) | HMDB | | 4,6,6-Trimethyl-bicyclo(3.1.1)hept-3-en-2-ol | HMDB | | 4,6,6-Trimethyl-bicyclo[3,1,1]hept-3-en-2-ol | HMDB | | 4,6,6-Trimethyl-bicyclo[3.1.1]hept-3-en-2-ol | HMDB | | 4,6,6-trimethylbicyclo(3.1.1)Hept-3-en-2-ol | HMDB | | 4,6,6-trimethylbicyclo[3.1.1]Hept-3-en-2-ol | HMDB | | 4,6,6-trimethylbicyclo[3.1.1]Hept-3-en-2-ol, 9ci | HMDB | | 4-Hydroxy-2,6,6-trimethylbicyclo(3.1.1)hept-2-ene | HMDB | | Berbenol | HMDB | | bicyclo(3.1.1)Hept-3-en-2-ol, 4,6,6-trimethyl- (9ci) | HMDB | | D-Verbenol | HMDB | | FEMA 3594 | HMDB | | PINEN-4-O1 | HMDB | | Pinen-4-ol | HMDB | | trans-Verbenol | HMDB | | Verbenol, (1S-(1alpha,2beta,5alpha))-isomer | MeSH, HMDB | | Verbenol, (1alpha,2alpha,5alpha)-isomer | MeSH, HMDB | | Verbenol, (1R-(1alpha,2beta,5alpha))-isomer | MeSH, HMDB | | Verbenol, (1S-(1alpha,2alpha,5alpha))-isomer | MeSH, HMDB | | Verbenol, (1R-(1alpha,2alpha,5alpha))-isomer | MeSH, HMDB | | Verbenol, (1alpha,2beta,5alpha)-isomer | MeSH, HMDB | | Verbenol | MeSH |
|
|---|
| Chemical Formula | C10H16O |
|---|
| Average Molecular Mass | 152.237 g/mol |
|---|
| Monoisotopic Mass | 152.120 g/mol |
|---|
| CAS Registry Number | 473-67-6 |
|---|
| IUPAC Name | 4,6,6-trimethylbicyclo[3.1.1]hept-3-en-2-ol |
|---|
| Traditional Name | verbenol |
|---|
| SMILES | CC1=CC(O)C2CC1C2(C)C |
|---|
| InChI Identifier | InChI=1S/C10H16O/c1-6-4-9(11)8-5-7(6)10(8,2)3/h4,7-9,11H,5H2,1-3H3 |
|---|
| InChI Key | WONIGEXYPVIKFS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bicyclic monoterpenoids. These are monoterpenoids containing exactly 2 rings, which are fused to each other. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Bicyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pinane monoterpenoid
- Bicyclic monoterpenoid
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f7c-6900000000-725a3ffa9a2c27657f75 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05ic-9220000000-b90f976faa3abdbac445 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0900000000-6ff630040559510e3f1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f79-0900000000-d77ee423e0f6f183d1df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kr-0900000000-bffee4e8536c2e4722c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-1384a080edbf08e60385 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-66f689eb5b9ce53ca27c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-0900000000-f5d698ddd2cc8c2502fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-c373c9eea3cebf186f53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-c373c9eea3cebf186f53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0900000000-c8f08741da2f9e0cc3d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-ed74c9ab4d3b693dc4fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f79-0900000000-4ea2325ddaf644f1dd2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014r-0900000000-688f35897c40c564f2a9 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036129 |
|---|
| FooDB ID | FDB014977 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00053892 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Verbenol |
|---|
| Chemspider ID | 55074 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61126 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|