| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:29:57 UTC |
|---|
| Update Date | 2016-11-09 01:13:40 UTC |
|---|
| Accession Number | CHEM007761 |
|---|
| Identification |
|---|
| Common Name | 2,2,6-TRIMETHYL-6-VINYLTETRAHYDROPYRAN |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of oxanes carrying a vinyl substituent at position 2 as well as three methyl substituents at positions 2, 6 and 6. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
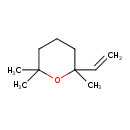
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,2,6-Trimethyl-6-vinyltetrahydro-2H-pyran | ChEBI | | 2,2,6-Trimethyl-6-vinyltetrahydropyran | ChEBI | | 2,6,6-Trimethyl-2-ethenyltetrahydro-2H-pyran | ChEBI | | 2,6,6-Trimethyl-2-ethenyltetrahydropyran | ChEBI | | 2,6,6-Trimethyl-2-vinyltetrahydropyran | ChEBI | | 2-Ethenyltetrahydro-2,6,6-trimethyl-2H-pyran | ChEBI | | 2-Vinyltetrahydro-2,6,6-trimethyl-2H-pyran | ChEBI | | Linalool 3,7-oxide | ChEBI | | Tetrahydro-2,2,6-trimethyl-6-vinyl-2H-pyran | ChEBI | | 2,2,6-Trimethyl-6-vinyl-tetrahydropyran | HMDB | | 2,6,6-Trimethyl-2-ethenyltetrahydro-2-pyran | HMDB | | 2,6,6-Trimethyl-2-vinyl-tetrahydropyrane | HMDB | | 2-ethenyltetrahydro-2,6,6-Trimethylpyran, 9ci | HMDB | | Dehydroxylinalool oxide a | HMDB | | FEMA 3735 | HMDB |
|
|---|
| Chemical Formula | C10H18O |
|---|
| Average Molecular Mass | 154.249 g/mol |
|---|
| Monoisotopic Mass | 154.136 g/mol |
|---|
| CAS Registry Number | 7392-19-0 |
|---|
| IUPAC Name | 2-ethenyl-2,6,6-trimethyloxane |
|---|
| Traditional Name | 2-ethenyl-2,6,6-trimethyloxane |
|---|
| SMILES | CC1(C)CCCC(C)(O1)C=C |
|---|
| InChI Identifier | InChI=1S/C10H18O/c1-5-10(4)8-6-7-9(2,3)11-10/h5H,1,6-8H2,2-4H3 |
|---|
| InChI Key | NETOHYFTCONTDT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oxanes. Oxanes are compounds containing an oxane (tetrahydropyran) ring, which is a six-member saturated aliphatic heterocycle with one oxygen atom and five carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Oxanes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Oxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxane
- Oxacycle
- Ether
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fwl-9300000000-bd4fe33bbcf6d0369e1f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2900000000-f9ecba55ccee79339306 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-9100000000-6c84d3d773b31f16dde0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05nf-9000000000-afc9e9075bde776980fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1900000000-e8e599e465eea0fbfb0d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-7900000000-00f419898f3a112d466e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06ds-9100000000-3350510ed9b6aae9292b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-d2363ae8d4dbdcccda80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-5900000000-67bec87194434c0dda41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-6900000000-aee78cdcdb533177a9bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05o3-9100000000-6d42ee439d476a67a5ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001l-9000000000-f3188b3d77123058e554 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9000000000-62fa83c27d8c6fac30d6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037133 |
|---|
| FooDB ID | FDB016128 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Linalool |
|---|
| Chemspider ID | 455792 |
|---|
| ChEBI ID | 132848 |
|---|
| PubChem Compound ID | 522514 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|