| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:29:40 UTC |
|---|
| Update Date | 2016-11-09 01:13:39 UTC |
|---|
| Accession Number | CHEM007735 |
|---|
| Identification |
|---|
| Common Name | 3,3,5-TRIMETHYLCYCLOHEXANOL |
|---|
| Class | Small Molecule |
|---|
| Description | A secondary alcohol that is cyclohexanol substituted by two methyl groups at the 3-position and one methyl group at the 5-position. It exhibits inhibitory activity against HMG-CoA reductase ( EC 1.1.1.34/EC 1.1.1.88). |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
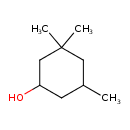
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,3,5-Trimethyl-1-cyclohexanol | ChEBI | | Homomenthol | ChEBI | | 1-Methyl-3-dimethylcyclohexanol-5 | HMDB | | 3,3,5-Trimethyl-(1R,5S)-rel-cyclohexanol | HMDB | | 3,3,5-Trimethyl-cis-cyclohexanol | HMDB | | 3,3,5-Trimethyl-cyclohexanol | HMDB | | 3,3,5-Trimethyl-trans-cyclohexanol | HMDB | | 3,3,5-Trimethylcyclohexanol (c,t) | HMDB | | 3,3,5-Trimethylcyclohexanol,camp t | HMDB | | 3,5,5-Trimethylcyclohexanol | HMDB | | cis-3,3,5-Trimethylcyclohexanol | HMDB | | Cyclonol | HMDB | | dihydro-Isophorol | HMDB | | FEMA 3962 | HMDB | | TMC | HMDB | | trans-3,3,5-Trimethylcyclohexanol | HMDB | | trans-3,5,5-Trimethylcyclohexan-1-ol | HMDB | | Trimethylcyclohexanol | HMDB | | Dihydroisophorol | ChEBI | | 3,3,5-Trimethylcyclohexanol, (cis)-isomer | MeSH | | 3,3,5-Trimethylcyclohexanol, (trans)-isomer | MeSH |
|
|---|
| Chemical Formula | C9H18O |
|---|
| Average Molecular Mass | 142.239 g/mol |
|---|
| Monoisotopic Mass | 142.136 g/mol |
|---|
| CAS Registry Number | 116-02-9 |
|---|
| IUPAC Name | 3,3,5-trimethylcyclohexan-1-ol |
|---|
| Traditional Name | 3,3,5-trimethylcyclohexanol |
|---|
| SMILES | CC1CC(O)CC(C)(C)C1 |
|---|
| InChI Identifier | InChI=1S/C9H18O/c1-7-4-8(10)6-9(2,3)5-7/h7-8,10H,4-6H2,1-3H3 |
|---|
| InChI Key | BRRVXFOKWJKTGG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cyclohexanols. Cyclohexanols are compounds containing an alcohol group attached to a cyclohexane ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Cyclohexanols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclohexanol
- Cyclic alcohol
- Hydrocarbon derivative
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9300000000-244f20d79cae6e7e51b3 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9300000000-244f20d79cae6e7e51b3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-005a-9300000000-d7f74ae639a6d7f7fe0d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-006t-9700000000-98677d3381cb7d575de1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0900000000-1b18235f590ccbf61da2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004l-3900000000-9026fc236779085d8ccf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05nb-9200000000-8c94db27037d76dff113 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-524e1240924affa73e1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-ea27540a52f91b77ca9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002g-9700000000-f11d1ab2b3606dbc2527 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-9400000000-a2c3605f4c1480924a6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200000000-2d34cfe7f27ebee54314 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0apl-9000000000-6b72ba755ce195c7f0a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-31a4c0b614cfbc7c3156 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-3962cd1a6f44a7191826 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0900000000-1bd0f7af6c59ba043f8e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029557 |
|---|
| FooDB ID | FDB000708 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 7997 |
|---|
| ChEBI ID | 59065 |
|---|
| PubChem Compound ID | 8298 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|