| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:28:45 UTC |
|---|
| Update Date | 2016-11-09 01:13:39 UTC |
|---|
| Accession Number | CHEM007663 |
|---|
| Identification |
|---|
| Common Name | THIOACETIC ACID |
|---|
| Class | Small Molecule |
|---|
| Description | A thioacetic acid that is acetic acid in which the oxygen atom of the hydroxy group has been replaced by a sulfur atom. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
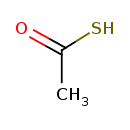
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Acetyl mercaptan | ChEBI | | CH3COSH | ChEBI | | Thioacetic acid | ChEBI | | Thioacetic S-acid | ChEBI | | Thioacetate | Generator | | Ethanethioate | Generator | | Ethanethioic O-acid | HMDB | | Ethanethiolic acid | HMDB | | Methanecarbothiolic acid | HMDB | | Schiff'S reagent | HMDB | | Thiacetic acid | HMDB | | Thio-acetic acid | HMDB | | Thioacetate esters | HMDB | | Thiolacetic acid | HMDB | | Thiolacetic acid? | HMDB | | Thionoacetic acid | HMDB | | Thioacetic acid, potassium salt | HMDB | | Thioacetic acid, sodium salt | HMDB |
|
|---|
| Chemical Formula | C2H4OS |
|---|
| Average Molecular Mass | 76.118 g/mol |
|---|
| Monoisotopic Mass | 75.998 g/mol |
|---|
| CAS Registry Number | 507-09-5 |
|---|
| IUPAC Name | ethanethioic S-acid |
|---|
| Traditional Name | schiff reagent |
|---|
| SMILES | CC(S)=O |
|---|
| InChI Identifier | InChI=1S/C2H4OS/c1-2(3)4/h1H3,(H,3,4) |
|---|
| InChI Key | DUYAAUVXQSMXQP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carbothioic s-acids. These are organic acids with the general formula RCS-OH (R=H, organic group). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carbothioic S-acids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Carbothioic S-acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carbothioic s-acid
- Carbodithioic acid
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Alkylthiol
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-9000000000-06248445f56db7f10761 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-9edb1da91d26c472db74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9000000000-d7b964960a1c1e4be5c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-2aed74d00cb791bf0dcc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dl-9000000000-3cb2ccfaeee23177c1c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-bdd638bc137bd61d2bc8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-9000000000-5bd7f1b88de4d4ef9be8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-9000000000-5f6f7fef7d8c01427470 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9000000000-01257c437988da882c19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-9633f3baf3f013462f5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-9000000000-b061934dee6f4e2506c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-9000000000-d222edf5c2b479bd176d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9000000000-537abdbe4f5b71b09ed6 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031188 |
|---|
| FooDB ID | FDB003208 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10052 |
|---|
| ChEBI ID | 16555 |
|---|
| PubChem Compound ID | 10484 |
|---|
| Kegg Compound ID | C01857 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=23298036 | | 2. Yang W, Du DM: Squaramide-catalysed enantio- and diastereoselective sulfa-Michael addition of thioacetic acid to alpha,beta-disubstituted nitroalkenes. Org Biomol Chem. 2012 Sep 14;10(34):6876-84. doi: 10.1039/c2ob26068a. Epub 2012 Jul 31. | | 3. Piro B, Zhang QD, Reisberg S, Noel V, Dang LA, Duc HT, Pham MC: Direct and rapid electrochemical immunosensing system based on a conducting polymer. Talanta. 2010 Jul 15;82(2):608-12. doi: 10.1016/j.talanta.2010.05.015. Epub 2010 May 19. | | 4. Liu M, Deng J, Lai C, Chen Q, Zhao Q, Zhang Y, Li H, Yao S: Synthesis, characterization of conjugated oligo-phenylene-ethynylenes and their supramolecular interaction with beta-cyclodextrin for salicylaldehyde detection. Talanta. 2012 Oct 15;100:229-38. doi: 10.1016/j.talanta.2012.08.008. Epub 2012 Aug 17. | | 5. Chauhan HP, Bhatiya S: Synthesis, spectroscopic, thermal and antimicrobial studies of toluene-3,4-dithiolatoarsenic(III) derivatives with some oxygen and sulphur donor ligands. Spectrochim Acta A Mol Biomol Spectrosc. 2012 Nov;97:1133-9. doi: 10.1016/j.saa.2012.07.086. Epub 2012 Aug 3. | | 6. Inoue K, Asai N, Mizuo H, Fukuda K, Kusano K, Yoshimura T: Unique metabolic pathway of [(14)C]lenvatinib after oral administration to male cynomolgus monkey. Drug Metab Dispos. 2012 Apr;40(4):662-70. doi: 10.1124/dmd.111.043281. Epub 2011 Dec 29. | | 7. Robertson F, Wu J: Convenient synthesis of allylic thioethers from phosphorothioate esters and alcohols. Org Lett. 2010 Jun 4;12(11):2668-71. doi: 10.1021/ol1009202. | | 8. Fischer JA, Zoldan VC, Benitez G, Rubert AA, Ramirez EA, Carro P, Salvarezza RC, Pasa AA, Vela ME: Sulfidization of Au(111) from thioacetic acid: an experimental and theoretical study. Langmuir. 2012 Oct 30;28(43):15278-85. doi: 10.1021/la303059u. Epub 2012 Oct 18. | | 9. Bendena WG, Zhang J, Burtenshaw SM, Tobe SS: Evidence for differential biosynthesis of juvenile hormone (and related) sesquiterpenoids in Drosophila melanogaster. Gen Comp Endocrinol. 2011 May 15;172(1):56-61. doi: 10.1016/j.ygcen.2011.02.014. Epub 2011 Feb 24. | | 10. Cimadevilla F, Garcia ME, Garcia-Vivo D, Ruiz MA, Graiff C, Tiripicchio A: Reactions of the tetrafluoroborate complex [Mo2Cp2(kappa(2)-F2BF2)(mu-PPh2)2(CO)]BF4 with mono- and bidentate ligands having E-H bonds (E = O, S, Se, N, P). Inorg Chem. 2012 Jul 2;51(13):7284-95. doi: 10.1021/ic300626y. Epub 2012 Jun 20. | | 11. Hintermann L, Turockin A: Reversible generation of metastable enols in the 1,4-addition of thioacetic acid to alpha,beta-unsaturated carbonyl compounds. J Org Chem. 2012 Dec 21;77(24):11345-8. doi: 10.1021/jo3021709. Epub 2012 Dec 3. | | 12. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|