| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:28:38 UTC |
|---|
| Update Date | 2016-11-09 01:13:38 UTC |
|---|
| Accession Number | CHEM007654 |
|---|
| Identification |
|---|
| Common Name | THEASPIRANE |
|---|
| Class | Small Molecule |
|---|
| Description | A norisoprenoid with forumula C13H22O that is a flavour component found in various essential oils such as raspberry oil and passion fruit oil. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
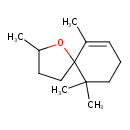
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Oxaspiro-2,6,10,10-tetramethyl[4.5]dec-6-ene | ChEBI | | 1-Oxaspiro-[4,5]-2,6,10,10-tetramethyl-6-decene | ChEBI | | FEMA 3774 | ChEBI | | 2,6,6,10-Tetramethyl-1-oxaspiro(4.5)dec-9-ene | MeSH | | (+/-)-theaspirane | HMDB | | 1-oxaspiro-2,6,10,10-Tetramethyl(4.5)dec-6-ene | HMDB | | 2,6,10,10-Tetramethyl-1-oxa-spiro[4.5]dec-6-ene | HMDB | | 2,6,10,10-Tetramethyl-1-oxaspiro(4.5)dec-6-ene | HMDB | | 2,6,10,10-Tetramethyl-1-oxaspiro[4.5]dec-6-ene | HMDB | | 2,6,10,10-Tetramethyl-1-oxaspiro[4.5]dec-6-ene, 9ci | HMDB | | 2,6,10,10-Tetramethyl-oxa-spiro-dec-6-ene | HMDB | | 6,9-Epoxy-4-megastigmene | HMDB | | cis-Theaspirane | HMDB | | Theaspirane a | HMDB | | Theaspirane b | HMDB | | Theaspirane is I | HMDB | | Theaspirane is II | HMDB | | Theaspirane, I | HMDB | | Theaspirane, II | HMDB |
|
|---|
| Chemical Formula | C13H22O |
|---|
| Average Molecular Mass | 194.313 g/mol |
|---|
| Monoisotopic Mass | 194.167 g/mol |
|---|
| CAS Registry Number | 36431-72-8 |
|---|
| IUPAC Name | 2,6,10,10-tetramethyl-1-oxaspiro[4.5]dec-6-ene |
|---|
| Traditional Name | 2,6,10,10-tetramethyl-1-oxaspiro[4.5]dec-6-ene |
|---|
| SMILES | CC1CCC2(O1)C(C)=CCCC2(C)C |
|---|
| InChI Identifier | InChI=1S/C13H22O/c1-10-6-5-8-12(3,4)13(10)9-7-11(2)14-13/h6,11H,5,7-9H2,1-4H3 |
|---|
| InChI Key | GYUZHTWCNKINPY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydrofurans. These are heterocyclic compounds containing a saturated, aliphatic, five-membered ring where a carbon is replaced by an oxygen. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydrofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydrofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydrofuran
- Oxacycle
- Ether
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0079-2900000000-2bb3f9923b0079e6cd6a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1900000000-51e382d753abc3960ba4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-5900000000-760acb81cc639794979d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ldl-9200000000-a2089cbe51cc1d7f302d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-e84c80ad2252bba16792 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-6ada400727fc5c863b4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pbi-3900000000-397f259370d23911c81c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-bb6971ce2781807265e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-6900000000-3a42b716d3f3705e38af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9200000000-f5385e4c728494f4972d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-f4ac9f2710ef63610b81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-82ca283a492052b65835 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0900000000-3f03637207802e999f02 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036823 |
|---|
| FooDB ID | FDB015771 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55810 |
|---|
| ChEBI ID | 89598 |
|---|
| PubChem Compound ID | 61953 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB16086 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|