| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:28:33 UTC |
|---|
| Update Date | 2016-11-09 01:13:38 UTC |
|---|
| Accession Number | CHEM007647 |
|---|
| Identification |
|---|
| Common Name | ALPHA-(P-(1,1,3,3-TETRAMETHYLBUTYL)PHENYL)-OMEGA-HYDROXYPOLY(OXYETHYLENE)(1 MOL) |
|---|
| Class | Small Molecule |
|---|
| Description | Pesticide diluent in foods |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
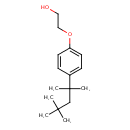
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Octoxinol | Kegg | | Polyoxysthylene mono ether | Kegg | | Triton X-100 | Kegg | | alpha-(4-(1,1,3,3-Tetramethylbutyl)phenyl)-omega-hydroxypoly(oxy-1,2-ethanediyl) | Kegg | | a-(4-(1,1,3,3-Tetramethylbutyl)phenyl)-omega-hydroxypoly(oxy-1,2-ethanediyl) | Generator | | Α-(4-(1,1,3,3-tetramethylbutyl)phenyl)-omega-hydroxypoly(oxy-1,2-ethanediyl) | Generator | | Octoxinols | MeSH | | Octoxynol | MeSH | | Octoxynol 9 | MeSH | | Octoxynol-9 | MeSH | | Octoxynols | MeSH | | Octylphenoxy polyethoxyethanol | MeSH | | Octylphenoxypolyethoxyethanols | MeSH | | Polyethoxyethanol, octylphenoxy | MeSH | | Triton X 100 | MeSH | | Triton X 305 | MeSH | | Triton X 45 | MeSH | | Triton X-305 | MeSH | | Triton X-45 | MeSH | | Triton X100 | MeSH | | Triton X305 | MeSH | | Triton X45 | MeSH |
|
|---|
| Chemical Formula | C16H26O2 |
|---|
| Average Molecular Mass | 250.382 g/mol |
|---|
| Monoisotopic Mass | 250.193 g/mol |
|---|
| CAS Registry Number | 2315-67-5 |
|---|
| IUPAC Name | 2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethan-1-ol |
|---|
| Traditional Name | triton X |
|---|
| SMILES | CC(C)(C)CC(C)(C)C1=CC=C(OCCO)C=C1 |
|---|
| InChI Identifier | InChI=1S/C16H26O2/c1-15(2,3)12-16(4,5)13-6-8-14(9-7-13)18-11-10-17/h6-9,17H,10-12H2,1-5H3 |
|---|
| InChI Key | JYCQQPHGFMYQCF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanes. These are organic compounds containing a phenylpropane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpropanes |
|---|
| Direct Parent | Phenylpropanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-2910000000-d88e1ec4af1bb49a1a97 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1290000000-4f4925ee673ca7d83c72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6t-6690000000-ac57603c5fb98daf3e80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06tv-4910000000-9d337d399fa4a37e3656 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-4efc65b5e7c177033f69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-6d15651eb5aeb5418e36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-1940000000-5155cafb399e8c226f68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0190000000-1b8ff08f87a4ea77303a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-4190000000-b2758df5192b4dc07a42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0900000000-7cc4792bf62c5d197287 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0259285 |
|---|
| FooDB ID | FDB017724 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 5388 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C11623 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|