| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:27:42 UTC |
|---|
| Update Date | 2016-11-09 01:13:37 UTC |
|---|
| Accession Number | CHEM007581 |
|---|
| Identification |
|---|
| Common Name | SUCCINIC ANHYDRIDE |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- IARC Carcinogens Group 3
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
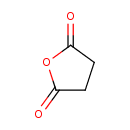
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,5-Diketotetrahydrofuran | ChEBI | | 2,5-Dioxotetrahydrofuran | ChEBI | | Bernsteinsaeureanhydrid | ChEBI | | Butanedioic anhydride | ChEBI | | Dihydro-2,5-furandione | ChEBI | | Dihydrofuran-2,5-dione | ChEBI | | Succinic acid anhydride | ChEBI | | Succinyl anhydride | ChEBI | | Succinyl oxide | ChEBI | | Tetrahydro-2,5-dioxofuran | ChEBI | | Tetrahydro-2,5-furandione | ChEBI | | Succinate anhydride | Generator | | 2,5(3H,4H)-Furandione | HMDB | | 2,5-Furandione, dihydro-, mono-C11-13-alkenyl derivs. | HMDB | | 2-Alkenyl (C11-C13) succinic acid anhydride | HMDB | | Butanedioic acid,anhydride succinic anhydride | HMDB | | Dihydro-2, 5-furandione | HMDB | | Dihydro-2,5-diketotetrahydrofuran | HMDB | | Dihydro-furan-2,5-dione | HMDB | | Oxolane-2,5-dione | HMDB | | Rikacid sa | HMDB | | SAA | HMDB | | Succinic anhydride treated bovine serum albumin | HMDB | | Succinic anhydride treated bsa | HMDB | | Succinyl peroxide | HMDB | | Succinyloxide | HMDB | | Tetrahydro-2, 5-dioxofuran | HMDB |
|
|---|
| Chemical Formula | C4H4O3 |
|---|
| Average Molecular Mass | 100.073 g/mol |
|---|
| Monoisotopic Mass | 100.016 g/mol |
|---|
| CAS Registry Number | 108-30-5 |
|---|
| IUPAC Name | oxolane-2,5-dione |
|---|
| Traditional Name | succinic anhydride |
|---|
| SMILES | O=C1CCC(=O)O1 |
|---|
| InChI Identifier | InChI=1S/C4H4O3/c5-3-1-2-4(6)7-3/h1-2H2 |
|---|
| InChI Key | RINCXYDBBGOEEQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dicarboxylic acids and derivatives. These are organic compounds containing exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Dicarboxylic acids and derivatives |
|---|
| Direct Parent | Dicarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dicarboxylic acid or derivatives
- Tetrahydrofuran
- Carboxylic acid anhydride
- Lactone
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kdi-9200000000-7aa5270b2ac5ed677ac4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2900000000-b6c9d054dced9b9c7d79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-4900000000-f15e0d8dde55d48b1642 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-751ce6ded2976303a3ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-fce94dd02c8af5927d04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-9000000000-64e15626f36dab29d672 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pb9-9000000000-d647422e00ac0f19d689 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-bd4c8170b9ee3c19f59a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-9000000000-c55763477e5bb8a6b707 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pb9-9000000000-a693fcab8c2aa97ff11c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zfr-9700000000-4d81f50d0ea48e8c13dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9100000000-23d4f9891f64c49c5789 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-58e61e6d42bb2d9c8fe8 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032523 |
|---|
| FooDB ID | FDB010338 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00034296 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Succinic anhydride |
|---|
| Chemspider ID | 7634 |
|---|
| ChEBI ID | 36595 |
|---|
| PubChem Compound ID | 7922 |
|---|
| Kegg Compound ID | C19524 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|