| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:27:14 UTC |
|---|
| Update Date | 2016-11-09 01:09:57 UTC |
|---|
| Accession Number | CHEM007551 |
|---|
| Identification |
|---|
| Common Name | SPIRO(2,4-DITHIA-1-METHYL-8-OXABICYCLO(3.3.0)OCTANE-3,3'-(1'-OXA-2'-METHYL)CYCLOPENTANE) |
|---|
| Class | Small Molecule |
|---|
| Description | Hexahydro-2',3alpha-dimethylspiro[1,3-dithiolo[4,5-b]furan-2,3'(2'H)-furan] is a major component (65%) of the meaty flavour ingredient *FEMA 3270*. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
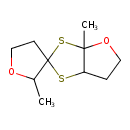
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Hexahydro-2',3a-dimethylspiro[1,3-dithiolo[4,5-b]furan-2,3'(2'H)-furan] | Generator | | Hexahydro-2',3α-dimethylspiro[1,3-dithiolo[4,5-b]furan-2,3'(2'H)-furan] | Generator |
|
|---|
| Chemical Formula | C10H16O2S2 |
|---|
| Average Molecular Mass | 232.363 g/mol |
|---|
| Monoisotopic Mass | 232.059 g/mol |
|---|
| CAS Registry Number | 38325-25-6 |
|---|
| IUPAC Name | 2',3a-dimethyl-tetrahydrospiro[[1,3]dithiolo[4,5-b]furan-2,3'-oxolane] |
|---|
| Traditional Name | 2',3a-dimethyl-dihydro-5H-spiro[[1,3]dithiolo[4,5-b]furan-2,3'-oxolane] |
|---|
| SMILES | CC1OCCC11SC2CCOC2(C)S1 |
|---|
| InChI Identifier | InChI=1S/C10H16O2S2/c1-7-10(4-6-11-7)13-8-3-5-12-9(8,2)14-10/h7-8H,3-6H2,1-2H3 |
|---|
| InChI Key | LGPJANFPSFABDB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monothioketals. Monothioketals are compounds containing a monothioketal functional group with the general structure R2C(OR')(SR') with (R = any atom but H). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Thioacetals |
|---|
| Sub Class | Monothioacetals |
|---|
| Direct Parent | Monothioketals |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dithioketal
- Monothioketal
- Thioacetal
- Dithiolane
- Tetrahydrofuran
- 1,3-dithiolane
- Thioether
- Ether
- Dialkyl ether
- Dialkylthioether
- Organoheterocyclic compound
- Oxacycle
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gb9-4950000000-24c75a1a478e3bb543cd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0aor-9520000000-f20d385507da45a44fc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2940000000-d70e0b612622d9e89b4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-5570274e78a958ad689d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1490000000-97e1902f5fb8e8c83f29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0159-7900000000-489273e89c8a851ab99d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bti-9200000000-194b520a557ebaad7b94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-6b9b34f3eb47fc4dd934 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00lr-0690000000-63469d1556d50a188aa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-7940000000-d7304c149ca21e72016e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-81f5c7c17e5ce456eac4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-2950000000-3446d44b25b07dcdd724 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0avr-9740000000-11f221b89b48c6b4d613 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037809 |
|---|
| FooDB ID | FDB016954 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55830 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61979 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|