| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:26:30 UTC |
|---|
| Update Date | 2016-11-09 01:09:56 UTC |
|---|
| Accession Number | CHEM007505 |
|---|
| Identification |
|---|
| Common Name | SODIUM GLUCONATE |
|---|
| Class | Small Molecule |
|---|
| Description | An organic sodium salt having D-gluconate as the counterion. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- OECD HPV Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| D-Gluconic acid, monosodium salt | ChEBI | | Gluconic acid sodium salt | ChEBI | | Monosodium D-gluconate | ChEBI | | Monosodium gluconate | ChEBI | | Sodium (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate | ChEBI | | D-Gluconate, monosodium salt | Generator | | Gluconate sodium salt | Generator | | Monosodium D-gluconic acid | Generator | | Monosodium gluconic acid | Generator | | Sodium (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoic acid | Generator | | Sodium gluconic acid | Generator | | Gluconic acid, fe(+2) salt, dihydrate | MeSH | | Gluconic acid, aluminum (3:1) salt | MeSH | | Gluconic acid, lanthanum(+3) salt | MeSH | | Gluconic acid, monolithium salt | MeSH | | D-Gluconic acid | MeSH | | Gluconic acid, (159)dysprosium-labeled salt | MeSH | | Gluconic acid, (99)technecium (5+) salt | MeSH | | Gluconic acid, 6-(14)C-labeled | MeSH | | Gluconic acid, copper salt | MeSH | | Gluconic acid, magnesium (2:1) salt | MeSH | | Gluconic acid, manganese (2:1) salt | MeSH | | Gluconic acid, zinc salt | MeSH | | Gluconic acid, cesium(+3) salt | MeSH | | Zinc gluconate | MeSH | | Gluconic acid, (14)C-labeled | MeSH | | Gluconic acid, 1-(14)C-labeled | MeSH | | Gluconic acid, cobalt (2:1) salt | MeSH | | Gluconic acid, tin(+2) salt | MeSH | | D-Gluconate | MeSH | | Boron gluconate | MeSH | | Gluconate | MeSH | | Gluconic acid | MeSH | | Gluconic acid, (113)indium-labeled | MeSH | | Gluconic acid, monosodium salt | MeSH | | Magnesium gluconate | MeSH | | Manganese gluconate | MeSH | | Gluconic acid, sodium salt | MeSH | | Lithium gluconate | MeSH | | Magnerot | MeSH | | Gluconic acid, calcium salt | MeSH | | Gluconic acid, monopotassium salt | MeSH | | Gluconic acid, strontium (2:1) salt | MeSH | | Gluconic acid, ammonium salt | MeSH | | Gluconic acid, potassium salt | MeSH |
|
|---|
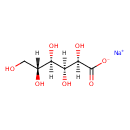
| Chemical Formula | C6H11NaO7 |
|---|
| Average Molecular Mass | 218.137 g/mol |
|---|
| Monoisotopic Mass | 218.040 g/mol |
|---|
| CAS Registry Number | 527-07-1 |
|---|
| IUPAC Name | sodium (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate |
|---|
| Traditional Name | sodium D-gluconate |
|---|
| SMILES | [Na+].[H][C@@](O)(CO)[C@@]([H])(O)[C@]([H])(O)[C@@]([H])(O)C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C6H12O7.Na/c7-1-2(8)3(9)4(10)5(11)6(12)13;/h2-5,7-11H,1H2,(H,12,13);/q;+1/p-1/t2-,3-,4+,5-;/m1./s1 |
|---|
| InChI Key | UPMFZISCCZSDND-JJKGCWMISA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gluconic_acid
- Medium-chain hydroxy acid
- Medium-chain fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Hydroxy acid
- Fatty acyl
- Fatty acid
- Monosaccharide
- Carboxylic acid salt
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Organic alkali metal salt
- Monocarboxylic acid or derivatives
- Polyol
- Organic sodium salt
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Carbonyl group
- Organic zwitterion
- Alcohol
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-3910000000-f9e103712665a44384d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-9500000000-3c306b8383712647b9b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08pv-9000000000-c861c860f4da7c8d327b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0kor-9700000000-ed4f798de632cb123241 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9300000000-034b93247d9adef5d466 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-c1f51fe041b8aa8f0f0a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT002440 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Sodium_gluconate |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 84997 |
|---|
| PubChem Compound ID | 84687 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|