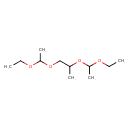| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:23:49 UTC |
|---|
| Update Date | 2016-11-09 01:09:54 UTC |
|---|
| Accession Number | CHEM007297 |
|---|
| Identification |
|---|
| Common Name | 1,2-(DI(1'-ETHOXY)ETHOXY)PROPANE |
|---|
| Class | Small Molecule |
|---|
| Description | 1,2-Bis(1-ethoxyethoxy)propane is a flavouring agent and adjuvant. Aldehyde generator used for enhancing the flavour of orange drinks. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Di((1'-ethoxy)ethoxy)propane | HMDB | | 1,2-Di((1-ethoxy)ethoxy)propane | HMDB | | 4,6,9-Trimethyl-3,5,8,10-tetraoxadodecane | HMDB | | 4,6,9-Trimethyl-3,5,8,10-tetraoxydodecane | HMDB | | 4,7,9-Trimethyl-3,5,8,10-tetraoxadodecane | HMDB | | FEMA 3534 | HMDB |
|
|---|
| Chemical Formula | C11H24O4 |
|---|
| Average Molecular Mass | 220.306 g/mol |
|---|
| Monoisotopic Mass | 220.167 g/mol |
|---|
| CAS Registry Number | 67715-79-1 |
|---|
| IUPAC Name | 4,6,9-trimethyl-3,5,8,10-tetraoxadodecane |
|---|
| Traditional Name | 4,6,9-trimethyl-3,5,8,10-tetraoxadodecane |
|---|
| SMILES | CCOC(C)OCC(C)OC(C)OCC |
|---|
| InChI Identifier | InChI=1S/C11H24O4/c1-6-12-10(4)14-8-9(3)15-11(5)13-7-2/h9-11H,6-8H2,1-5H3 |
|---|
| InChI Key | TZVJNJVDGXFMCF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acetals. Acetals are compounds having the structure R2C(OR')2 ( R' not Hydrogen) and thus diethers of geminal diols. Originally, the term was confined to derivatives of aldehydes (one R = H), but it now applies equally to derivatives of ketones (neither R = H ). Mixed acetals have different R' groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Ethers |
|---|
| Direct Parent | Acetals |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acetal
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0631-8910000000-dc3adbb5aeb7c5eb1c5d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dj-4940000000-4786313f2a20311620a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9600000000-d4712b13974d5fe28ab6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fs-9200000000-64f979f5d7e795146606 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00xr-8960000000-ed3a9fe65fef64149090 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000j-9510000000-b48de408132390f51c9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006t-9400000000-b5b12e41bd7f61d8815f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004r-5900000000-18ce0292cb4666a334a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-009l-9400000000-3e22cb4a726906677a8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zmu-9300000000-81c39be658a227099765 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-9300000000-d61fe866684054486864 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0flc-9500000000-462f5ed4408036867fb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006t-9000000000-fb59e0dcc09ab2db53a8 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037163 |
|---|
| FooDB ID | FDB016158 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4515081 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5362566 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|