| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:22:48 UTC |
|---|
| Update Date | 2016-11-09 01:09:53 UTC |
|---|
| Accession Number | CHEM007225 |
|---|
| Identification |
|---|
| Common Name | PIPERONYL ACETATE |
|---|
| Class | Small Molecule |
|---|
| Description | Piperonyl acetate is found in green vegetables. Piperonyl acetate is a flavouring agent. Piperonyl acetate is present in endive (Cichorium endiva |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
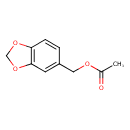
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Piperonyl acetic acid | Generator | | (3,4-Methylenedioxy)benzyl acetate | HMDB | | 1,3-Benzodioxol, 5-(acetoxymethyl) | HMDB | | 1,3-Benzodioxol-5-ylmethyl acetate | HMDB | | 1,3-Benzodioxole-5-methanol, 5-acetate | HMDB | | 1,3-Benzodioxole-5-methanol, acetate | HMDB | | 3,4-Methylenedioxybenzyl acetate | HMDB | | Acetic acid, (3, 4-methylenedioxy)benzyl ester | HMDB | | Acetic acid, (3,4-methylenedioxy)benzyl ester | HMDB | | FEMA 2912 | HMDB | | Heliotropin acetate | HMDB | | Heliotropyl acetate | HMDB | | Methyl 1,3-benzodioxol-5-ylacetate | HMDB | | Piperonal acetate | HMDB | | Piperonyl alcohol, acetate | HMDB | | Piperonyl alcohol, acetate (6ci,7ci,8ci) | HMDB | | Piperonylacetate | HMDB | | (2H-1,3-Benzodioxol-5-yl)methyl acetic acid | Generator | | 1,3-Benzodioxole-5-methanol acetate | MeSH | | Piperonyl acetate | MeSH |
|
|---|
| Chemical Formula | C10H10O4 |
|---|
| Average Molecular Mass | 194.184 g/mol |
|---|
| Monoisotopic Mass | 194.058 g/mol |
|---|
| CAS Registry Number | 326-61-4 |
|---|
| IUPAC Name | 2H-1,3-benzodioxol-5-ylmethyl acetate |
|---|
| Traditional Name | piperonyl acetate |
|---|
| SMILES | CC(=O)OCC1=CC2=C(OCO2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C10H10O4/c1-7(11)12-5-8-2-3-9-10(4-8)14-6-13-9/h2-4H,5-6H2,1H3 |
|---|
| InChI Key | PFWYHTORQZAGCA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzodioxoles. These are organic compounds containing a benzene ring fused to either isomers of dioxole. Dioxole is a five-membered unsaturated ring of two oxygen atoms and three carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzodioxoles |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzodioxoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzodioxole
- Benzenoid
- Carboxylic acid ester
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Acetal
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0f76-4900000000-92da8e820031602709c7 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0f76-4900000000-92da8e820031602709c7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-4900000000-e40b664e869f3f27ae75 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-fae17415db827f864dab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-466494e8eb86c24650dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9800000000-ba12bb6a6e41a820a746 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2900000000-4620fb177da88172db65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-2900000000-540e08888772c4194626 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9500000000-0280106f7a7edf9f86d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-4aa46a9717b95d9ed721 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-44a492319107ab04e6ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9600000000-694e5b64c4e6746504ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-1750905022a298264c01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-0900000000-f2187ed17caab45933a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-5900000000-af92226ec7c8bc1af18d | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032614 |
|---|
| FooDB ID | FDB010555 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057346 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9101 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9473 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|