| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:19:44 UTC |
|---|
| Update Date | 2016-11-09 01:09:50 UTC |
|---|
| Accession Number | CHEM006947 |
|---|
| Identification |
|---|
| Common Name | OCTAHYDRO-4,8A-DIMETHYL-4A(2H)-NAPHTHOL |
|---|
| Class | Small Molecule |
|---|
| Description | Geosmin is found in corn. Implicated in off-flavour of shellfish, freshwater fish, drinking water and some vegetables.Geosmin, which literally translates to "earth smell", is an organic compound with a distinct earthy flavour and aroma, and is responsible for the earthy taste of beets and a contributor to the strong scent that occurs in the air when rain falls after a dry spell of weather (petrichor) or when soil is disturbed. The human nose is extremely sensitive to geosmin and is able to detect it at concentrations as low as 5 parts per trillion. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
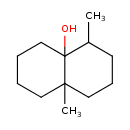
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,10-Dimethyl-9-decalol | HMDB | | 11,12,13-Trinor-5-eudesmanol | HMDB | | 4,8a-dimethyloctahydro-4a(2H)-Naphthalenol | HMDB | | 4,8alpha-Dimethyl-octahydro-naphthalen-4alpha-ol | HMDB | | octahydro-4,8a-Dimethyl-4a(2H)-naphthalenol, 9ci | HMDB |
|
|---|
| Chemical Formula | C12H22O |
|---|
| Average Molecular Mass | 182.303 g/mol |
|---|
| Monoisotopic Mass | 182.167 g/mol |
|---|
| CAS Registry Number | 23333-91-7 |
|---|
| IUPAC Name | 4,8a-dimethyl-decahydronaphthalen-4a-ol |
|---|
| Traditional Name | geosmin |
|---|
| SMILES | CC1CCCC2(C)CCCCC12O |
|---|
| InChI Identifier | InChI=1S/C12H22O/c1-10-6-5-8-11(2)7-3-4-9-12(10,11)13/h10,13H,3-9H2,1-2H3 |
|---|
| InChI Key | JLPUXFOGCDVKGO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tertiary alcohols. Tertiary alcohols are compounds in which a hydroxy group, -OH, is attached to a saturated carbon atom R3COH (R not H ). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Tertiary alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tertiary alcohol
- Cyclic alcohol
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udr-1900000000-73211b3533617178344c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01tj-3490000000-3ed4f0a4110d091adc8e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-0900000000-f6d5dbada1d48ea6ad2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-2900000000-ced0e0d8d0358c61d547 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059l-6900000000-380ea2bfec405ce5f64b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-f5e30aaf3cdff12d77a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000000-4a166ae95fb9e5300392 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gc9-1900000000-08145a39383b88307efd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-42496e3dcea777318d2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-2900000000-d36109084630437fa1a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9000000000-2131b82b958f6d9e7989 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-2d87f53c30e614a48654 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000000-2d87f53c30e614a48654 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0900000000-ad67dd9ba98bb5660914 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036461 |
|---|
| FooDB ID | FDB015352 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00013224 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Geosmin |
|---|
| Chemspider ID | 1176 |
|---|
| ChEBI ID | 46702 |
|---|
| PubChem Compound ID | 1213 |
|---|
| Kegg Compound ID | C16286 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|