| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:18:48 UTC |
|---|
| Update Date | 2016-11-09 01:09:49 UTC |
|---|
| Accession Number | CHEM006877 |
|---|
| Identification |
|---|
| Common Name | NERYL PROPIONATE |
|---|
| Class | Small Molecule |
|---|
| Description | Geranyl propionate is found in citrus. Geranyl propionate is found in citrus peel oils, kumquat peel oil, muscadine grape (Vitis rotundifolia), hop oil and cardamon (Ellettaria cardamomum). Geranyl propionate is a flavouring agent. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
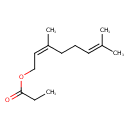
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Neryl propionic acid | Generator | | (Z)-3,7-Dimethyl-2,6-octadienyl propanoate | HMDB | | (Z)-3,7-Dimethyl-2,6-octadienyl propionate | HMDB | | 3,7-Dimethyl-1-propanoate(2Z)-2,6-octadien-1-ol | HMDB | | 3,7-Dimethyl-propanoate(2Z)-2,6-octadien-1-ol | HMDB | | 3,7-Dimethyl-propanoate(Z)-2,6-octadien-1-ol | HMDB | | 3,7-Dimethyl-propionate(Z)-2,6-octadien-1-ol | HMDB | | cis-3,7-Dimethyl-2,6-octadien-1-ol, propionate | HMDB | | cis-3,7-Dimethyl-2,6-octadien-1-yl propanoate | HMDB | | cis-3,7-Dimethyl-2,6-octadien-1-yl propionate | HMDB | | FEMA 2777 | HMDB | | Neryl propanoate | HMDB | | Propionic acid, 3,7-dimethyl-2,6-octadien-1-yl ester | HMDB | | Propionic acid, neryl ester | HMDB | | Geranyl propionic acid | Generator |
|
|---|
| Chemical Formula | C13H22O2 |
|---|
| Average Molecular Mass | 210.313 g/mol |
|---|
| Monoisotopic Mass | 210.162 g/mol |
|---|
| CAS Registry Number | 105-91-9 |
|---|
| IUPAC Name | (2Z)-3,7-dimethylocta-2,6-dien-1-yl propanoate |
|---|
| Traditional Name | (2Z)-3,7-dimethylocta-2,6-dien-1-yl propanoate |
|---|
| SMILES | CCC(=O)OC\C=C(\C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C13H22O2/c1-5-13(14)15-10-9-12(4)8-6-7-11(2)3/h7,9H,5-6,8,10H2,1-4H3/b12-9- |
|---|
| InChI Key | BYCHQEILESTMQU-XFXZXTDPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty alcohol esters. These are ester derivatives of a fatty alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohol esters |
|---|
| Direct Parent | Fatty alcohol esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty alcohol ester
- Monoterpenoid
- Acyclic monoterpenoid
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-2e0f1e77d40cf1eab2f5 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-2e0f1e77d40cf1eab2f5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ar9-9600000000-f257fb5330fa8c38781d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fr-5950000000-83906ea8eec63eda8ee7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-9600000000-4cec341190355ebaa8e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ldi-9100000000-681f954b00ebadab9980 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9370000000-9188f78aa531d9f5bc6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-9200000000-d31065d656fbf543fac7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-9300000000-45e472aa108728329088 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9300000000-8c6733edff435e342e53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9200000000-02cab8df7e1c56ff2c3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-f2eb9f46fa4edbed46cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9010000000-959987d3f6d69d861b4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9100000000-01494857f19fc13a4cf5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9000000000-7cb718f1188e4e255c8d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038258 |
|---|
| FooDB ID | FDB018004 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056009 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4517915 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5365982 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|