| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:18:47 UTC |
|---|
| Update Date | 2016-11-09 01:09:49 UTC |
|---|
| Accession Number | CHEM006874 |
|---|
| Identification |
|---|
| Common Name | NERYL FORMATE |
|---|
| Class | Small Molecule |
|---|
| Description | Neryl formate is found in lemon. Neryl formate is a food flavouring. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
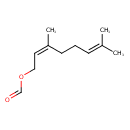
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Neryl formic acid | Generator | | (2E)-3,7-Dimethyl-2,6-octadien-1-ol, 1-formate | HMDB | | (2E)-3,7-Dimethyl-2,6-octadienyl formate | HMDB | | (2E)-3,7-Dimethylocta-2,6-dien-1-yl formate | HMDB | | (e)-3,7-Dimethyl-2,6-octadienyl formate | HMDB | | (e)-Geranyl formate | HMDB | | 3,7-Dimethyl-1-formate(2E)-2,6-octadien-1-ol | HMDB | | 3,7-Dimethyl-2,6-octadienyl ester(e)-formic acid | HMDB | | 3,7-Dimethyl-formate(2E)-2,6-octadien-1-ol | HMDB | | 3,7-Dimethyl-formate(e)-2,6-octadien-1-ol | HMDB | | FEMA 2514 | HMDB | | Formic acid, geraniol ester | HMDB | | Geraniol formate | HMDB | | Geranyl methanoate | HMDB | | trans-3, 7-Dimethyl-2,6-octadien-1-yl formate | HMDB | | trans-3,7-Dimethyl-2,6-octadien-1-ol formate | HMDB | | trans-3,7-Dimethyl-2,6-octadien-1-yl formate | HMDB | | trans-3,7-Dimethyl-2,6-octadien-1-yl methanoate | HMDB | | (Z)-3,7-Dimethyl-2,6-octadienyl formic acid | Generator | | Neryl formate | MeSH |
|
|---|
| Chemical Formula | C11H18O2 |
|---|
| Average Molecular Mass | 182.259 g/mol |
|---|
| Monoisotopic Mass | 182.131 g/mol |
|---|
| CAS Registry Number | 2142-94-1 |
|---|
| IUPAC Name | (2Z)-3,7-dimethylocta-2,6-dien-1-yl formate |
|---|
| Traditional Name | (2Z)-3,7-dimethylocta-2,6-dien-1-yl formate |
|---|
| SMILES | CC(C)=CCC\C(C)=C/COC=O |
|---|
| InChI Identifier | InChI=1S/C11H18O2/c1-10(2)5-4-6-11(3)7-8-13-9-12/h5,7,9H,4,6,8H2,1-3H3/b11-7- |
|---|
| InChI Key | FQMZVFJYMPNUCT-XFFZJAGNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty alcohol esters. These are ester derivatives of a fatty alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohol esters |
|---|
| Direct Parent | Fatty alcohol esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty alcohol ester
- Monoterpenoid
- Acyclic monoterpenoid
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9000000000-279f1c08bc764167c2f4 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9000000000-279f1c08bc764167c2f4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-9500000000-f6d47fb49d3834afd61d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0019-1900000000-06d3c97a8ca0a1cc8d1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-7900000000-3bfc9be480877bab35e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9100000000-28bc37ea6abd65905000 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1900000000-ade5c751ea0a3e26ed3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000x-7900000000-9eaa4f1b629cf5736854 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-171cc34cdc7bca93e068 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kr-3900000000-868c0bbad6e5e2b8d616 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9100000000-cdc566fe5ffa0c9ffc61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003i-9800000000-2d5268d90f70a9b33bc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9400000000-bfb585f4bf75db05f0ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053r-9200000000-33ab82b40fbcbfe30062 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9000000000-37e6869678099c1891c0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035156 |
|---|
| FooDB ID | FDB020002 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4510981 |
|---|
| ChEBI ID | 31648 |
|---|
| PubChem Compound ID | 5354882 |
|---|
| Kegg Compound ID | C12294 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|