| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:18:40 UTC |
|---|
| Update Date | 2016-11-09 01:09:49 UTC |
|---|
| Accession Number | CHEM006867 |
|---|
| Identification |
|---|
| Common Name | NEOHESPERIDIN DIHYDROCHALCONE |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the dihydrochalcones that is 3,2',4',6'-tetrahydroxy-4-methoxydihydrochalcone attached to a neohesperidosyl residue at position 4' via glycosidic linkage. It is found in sweet orange. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
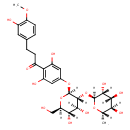
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Neohesperidine DC | MeSH | | Neohesperidine dihydrochalcone | MeSH |
|
|---|
| Chemical Formula | C28H36O15 |
|---|
| Average Molecular Mass | 612.581 g/mol |
|---|
| Monoisotopic Mass | 612.205 g/mol |
|---|
| CAS Registry Number | 20702-77-6 |
|---|
| IUPAC Name | 1-(4-{[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-2-yl]oxy}-2,6-dihydroxyphenyl)-3-(3-hydroxy-4-methoxyphenyl)propan-1-one |
|---|
| Traditional Name | neohesperidin dihydrochalcone |
|---|
| SMILES | [H][C@@]1(C)O[C@@]([H])(O[C@@]2([H])[C@]([H])(OC3=CC(O)=C(C(=O)CCC4=CC(O)=C(OC)C=C4)C(O)=C3)O[C@]([H])(CO)[C@@]([H])(O)[C@]2([H])O)[C@]([H])(O)[C@]([H])(O)[C@@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C28H36O15/c1-11-21(34)23(36)25(38)27(40-11)43-26-24(37)22(35)19(10-29)42-28(26)41-13-8-16(32)20(17(33)9-13)14(30)5-3-12-4-6-18(39-2)15(31)7-12/h4,6-9,11,19,21-29,31-38H,3,5,10H2,1-2H3/t11-,19+,21-,22+,23+,24-,25+,26+,27-,28+/m0/s1 |
|---|
| InChI Key | ITVGXXMINPYUHD-CUVHLRMHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alkyl-phenylketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl-phenylketone
- Acetophenone
- Nitrobenzene
- Nitroaromatic compound
- Benzoyl
- Aryl alkyl ketone
- Monocyclic benzene moiety
- Benzenoid
- C-nitro compound
- Organic nitro compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic oxoazanium
- Organopnictogen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 6V, Negative | splash10-0udi-0009001000-a784d35122c71c756b90 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 30V, Negative | splash10-03di-0001009000-828aa8353246f5f4043d | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 40V, Negative | splash10-0udi-0009103000-97bc3acb4370bf9313c9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-0w29-0119005000-c793e997a188821d0f59 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT 42V, positive | splash10-0udi-0000960000-472e33b531c61b98300c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05mk-0348932000-df6cd9b375db7056eae0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0649400000-2a887032b44aa75c8d3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pbi-0936000000-bd32f06b0c49d82b7fba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ik9-4529746000-3a493f7c0089f7f95e3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w2j-2936510000-2937915e513b94cadbe5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-9758000000-8ebac12374f6c970b439 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Neohesperidin_dihydrochalcone |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 83535 |
|---|
| PubChem Compound ID | 30231 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|