| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:17:28 UTC |
|---|
| Update Date | 2016-11-09 01:09:48 UTC |
|---|
| Accession Number | CHEM006769 |
|---|
| Identification |
|---|
| Common Name | METHYL 2-THIOFUROATE |
|---|
| Class | Small Molecule |
|---|
| Description | Methyl 2-thiofuroate is a flavouring ingredient with a fried, cooked onion odour. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
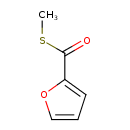
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 2-thiofuroic acid | Generator | | 2-Furancarbothioic acid, S-methyl ester | HMDB | | 2-Furoic acid, thio-, S-methyl ester | HMDB | | FEMA 3311 | HMDB | | Methyl thio-2-furoate | HMDB | | Methyl thiofuroate | HMDB | | Methylthiol furoate | HMDB | | S-Methyl 2-furancarbothioate | HMDB | | S-Methyl thio-2-furoate | HMDB | | S-Methyl thiofuroate | HMDB | | (Furan-2-yl)(methylsulphanyl)methanone | Generator |
|
|---|
| Chemical Formula | C6H6O2S |
|---|
| Average Molecular Mass | 142.176 g/mol |
|---|
| Monoisotopic Mass | 142.009 g/mol |
|---|
| CAS Registry Number | 13679-61-3 |
|---|
| IUPAC Name | furan-2-yl(methylsulfanyl)methanone |
|---|
| Traditional Name | furan-2-yl(methylsulfanyl)methanone |
|---|
| SMILES | CSC(=O)C1=CC=CO1 |
|---|
| InChI Identifier | InChI=1S/C6H6O2S/c1-9-6(7)5-3-2-4-8-5/h2-4H,1H3 |
|---|
| InChI Key | ISKUAGFDTRLBHG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as furoic acid and derivatives. These are aromatic heterocyclic compounds containing a furan ring, which carries a carboxyl group or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Furans |
|---|
| Sub Class | Furoic acid and derivatives |
|---|
| Direct Parent | Furoic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Furoic acid or derivatives
- Heteroaromatic compound
- Carbothioic s-ester
- Thiocarboxylic acid ester
- Oxacycle
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000b-9000000000-070d9ad5ca08deb8d309 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-b3e7894320302d0268f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-1900000000-64ee88f5a9d5d1021229 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9400000000-a58646d0ada4ad942926 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-633a2463afa0c1e55dba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-6900000000-42d47dbc65f779a99718 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014m-9000000000-0d0f301e8d53efd5a3c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kf-9600000000-fa8fc7376506197b0b77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-9000000000-d2ceed17c514d844ed70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-9000000000-9371a0ec97bf90209aa4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9200000000-ed062d6347fb7c59386e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-36a8a591bc1da1896e43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-3f7f696132aab0fba824 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037762 |
|---|
| FooDB ID | FDB016903 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55567 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61662 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|