| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:17:21 UTC |
|---|
| Update Date | 2016-11-09 01:09:48 UTC |
|---|
| Accession Number | CHEM006757 |
|---|
| Identification |
|---|
| Common Name | 2-METHYL-3-THIOACETOXY-4,5-DIHYDROFURAN |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of dihydrofurans that is 4,5-dihydrofuran substituted at positions 2 and 3 by methyl and thioacetoxy groups respectively. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
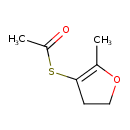
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Methyl-3-(thioacetoxy)-4,5-dihydrofuran | ChEBI | | 2-Methyl-3-thioacetoxy-4,5-dihydrofuran | ChEBI | | 2-Methyl-4,5-dihydro-3-furanthiol acetate | ChEBI | | 4,5-Dihydro-2-methyl-3-furanthiyl acetate | ChEBI | | Ethanethioic acid, S-(4,5-dihydro-2-methyl-3-furanyl) ester | ChEBI | | S-(4,5-Dihydro-2-methyl-3-furyl) thioacetate | ChEBI | | 2-Methyl-4,5-dihydro-3-furanthiol acetic acid | Generator | | 4,5-Dihydro-2-methyl-3-furanthiyl acetic acid | Generator | | Ethanethioate, S-(4,5-dihydro-2-methyl-3-furanyl) ester | Generator | | S-(4,5-Dihydro-2-methyl-3-furyl) thioacetic acid | Generator | | S-(4,5-Dihydro-2-methyl-3-furanyl) ethanethioic acid | Generator | | 3-Furanthiol, 4,5-dihydro-2-methyl-, acetate | HMDB | | Acetic acid, thio-, S-(4,5-dihydro-2-methyl-3-furyl) ester | HMDB | | FEMA 3636 | HMDB | | S-(4,5-dihydro-2-Methyl-3-furanyl) ethanethioate, 9ci | HMDB | | S-(4,5-dihydro-2-Methyl-3-furyl) ethanethioate | HMDB |
|
|---|
| Chemical Formula | C7H10O2S |
|---|
| Average Molecular Mass | 158.218 g/mol |
|---|
| Monoisotopic Mass | 158.040 g/mol |
|---|
| CAS Registry Number | 26486-14-6 |
|---|
| IUPAC Name | 1-[(2-methyl-4,5-dihydrofuran-3-yl)sulfanyl]ethan-1-one |
|---|
| Traditional Name | 1-[(2-methyl-4,5-dihydrofuran-3-yl)sulfanyl]ethanone |
|---|
| SMILES | CC(=O)SC1=C(C)OCC1 |
|---|
| InChI Identifier | InChI=1S/C7H10O2S/c1-5-7(3-4-9-5)10-6(2)8/h3-4H2,1-2H3 |
|---|
| InChI Key | YDYAMYYOQBGPRX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydrofurans. Dihydrofurans are compounds containing a dihydrofuran moiety, which is a furan derivative with only one double bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dihydrofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Dihydrofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydrofuran
- Carbothioic s-ester
- Thiocarboxylic acid ester
- Oxacycle
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kf-9400000000-7abb21deca903158577a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066r-2900000000-afde3190cbc0e1232f70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aou-3900000000-8141351d990508cb0051 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9000000000-151368e2442d0679f4bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1900000000-f10015ecf5b885fb820f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-4900000000-0fcc52038de8c8a5ce3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9200000000-195f865644fd3f395645 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0aou-3900000000-30cb399810f29ba5c4fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-9600000000-cbc11b0384bdadeeeaa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-9200000000-b527bb54740c43942043 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00ec-9000000000-1481aa1d0b29c4af4e50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01bc-9600000000-5381adc194bf41f7a870 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-9200000000-b50e8ccb0e2fd8d62216 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037786 |
|---|
| FooDB ID | FDB016930 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 20127092 |
|---|
| ChEBI ID | 131456 |
|---|
| PubChem Compound ID | 20831821 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|