| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:17:18 UTC |
|---|
| Update Date | 2016-11-09 01:09:48 UTC |
|---|
| Accession Number | CHEM006751 |
|---|
| Identification |
|---|
| Common Name | 2-(4-METHYL-5-THIAZOLYL)ETHYL HEXANOATE |
|---|
| Class | Small Molecule |
|---|
| Description | 2-(4-Methyl-5-thiazolyl)ethyl hexanoate is used as a food additive [EAFUS] ("EAFUS: Everything Added to Food in the United States. [http://www.eafus.com/]") |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
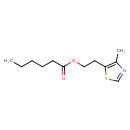
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(4-Methyl-5-thiazolyl)ethyl hexanoic acid | Generator | | 2-(4-Methylthiazol-5-yl)ethyl hexanoate | HMDB | | 2-(4-Methyl-1,3-thiazol-5-yl)ethyl hexanoic acid | Generator |
|
|---|
| Chemical Formula | C12H19NO2S |
|---|
| Average Molecular Mass | 241.350 g/mol |
|---|
| Monoisotopic Mass | 241.114 g/mol |
|---|
| CAS Registry Number | 94159-32-7 |
|---|
| IUPAC Name | 2-(4-methyl-1,3-thiazol-5-yl)ethyl hexanoate |
|---|
| Traditional Name | 2-(4-methyl-1,3-thiazol-5-yl)ethyl hexanoate |
|---|
| SMILES | CCCCCC(=O)OCCC1=C(C)N=CS1 |
|---|
| InChI Identifier | InChI=1S/C12H19NO2S/c1-3-4-5-6-12(14)15-8-7-11-10(2)13-9-16-11/h9H,3-8H2,1-2H3 |
|---|
| InChI Key | VJULDCZELAIZHC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 4,5-disubstituted thiazoles. 4,5-disubstituted thiazoles are compounds containing a thiazole ring substituted at positions 4 and 5 only. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Thiazoles |
|---|
| Direct Parent | 4,5-disubstituted thiazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4,5-disubstituted 1,3-thiazole
- Fatty acid ester
- Fatty acyl
- Heteroaromatic compound
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Azacycle
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0096-9600000000-dd1876ef7e10602b3f57 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-5490000000-da84d83323cedebb87d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-8910000000-3f791e2bfc685f534f7f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6u-9200000000-c3327e9308f06f290551 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0007-8590000000-9b2ab6d558bfae578561 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-9610000000-e37d5f051ae29cf56c83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mn-9200000000-b282c968bf552a8b50d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2920000000-e8f4325f514401361e64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fs-6900000000-d62a28c41c0aca030f39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-9500000000-333a172fc77dd3f43266 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0590000000-e023308443bd4f085843 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01r6-4900000000-540e77756652cbcf5f34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-5900000000-377ab810d3cf31314873 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032421 |
|---|
| FooDB ID | FDB009864 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 2289907 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3023839 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|