| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:16:07 UTC |
|---|
| Update Date | 2016-11-09 01:09:46 UTC |
|---|
| Accession Number | CHEM006638 |
|---|
| Identification |
|---|
| Common Name | METHYL N-METHYLANTHRANILATE |
|---|
| Class | Small Molecule |
|---|
| Description | A methyl ester resulting from the formal condensation of the carboxy group of N-methylanthranilic acid with methanol. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
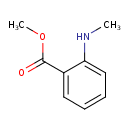
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Methylaminobenzoic acid methyl ester | ChEBI | | 2-Methylaminomethyl benzoate | ChEBI | | Dimethyl anthranilate | ChEBI | | Methyl methanthranilate | ChEBI | | Methyl methylaminobenzoate | ChEBI | | Methyl methylanthranilate | ChEBI | | Methyl N-methyl anthranylate | ChEBI | | N-Methylanthranilic acid, methyl ester | ChEBI | | 2-Methylaminobenzoate methyl ester | Generator | | 2-Methylaminomethyl benzoic acid | Generator | | Dimethyl anthranilic acid | Generator | | Methyl methanthranilic acid | Generator | | Methyl methylaminobenzoic acid | Generator | | Methyl methylanthranilic acid | Generator | | Methyl N-methyl anthranylic acid | Generator | | N-Methylanthranilate, methyl ester | Generator | | Methyl N-methylanthranilic acid | Generator | | 2-methylamino-Benzoic acid methyl ester | HMDB | | Anthranilic acid, N-methyl-, methyl ester | HMDB | | Benzoic acid, 2-(methylamino)-, methyl ester | HMDB | | FEMA 2718 | HMDB | | Methyl 2-(methylamino)benzoate | HMDB | | Methyl 2-methylaminobenzoate | HMDB | | Methyl benzoate, 2-methylamino | HMDB | | Methyl N-methyl anthranilate | HMDB | | Methyl N-methyl-O-anthranilate | HMDB | | Methyl O-(methylamino)benzoate | HMDB | | Methyl-2-(N-methylamino)benzoate | HMDB | | Methyl-N-methylanthranilate | HMDB | | N-Methyl methyl anthranilate | HMDB | | Methyl 2-(methylamino)benzoic acid | Generator | | Methyl N-methylanthranilate | MeSH |
|
|---|
| Chemical Formula | C9H11NO2 |
|---|
| Average Molecular Mass | 165.189 g/mol |
|---|
| Monoisotopic Mass | 165.079 g/mol |
|---|
| CAS Registry Number | 85-91-6 |
|---|
| IUPAC Name | methyl 2-(methylamino)benzoate |
|---|
| Traditional Name | methyl 2-(methylamino)benzoate |
|---|
| SMILES | CNC1=CC=CC=C1C(=O)OC |
|---|
| InChI Identifier | InChI=1S/C9H11NO2/c1-10-8-6-4-3-5-7(8)9(11)12-2/h3-6,10H,1-2H3 |
|---|
| InChI Key | GVOWHGSUZUUUDR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzoic acid esters. These are ester derivatives of benzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Benzoic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzoic acid or derivatives
- Benzoate ester
- Benzoyl
- Aniline or substituted anilines
- Phenylalkylamine
- Secondary aliphatic/aromatic amine
- Vinylogous amide
- Methyl ester
- Carboxylic acid ester
- Amino acid or derivatives
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Secondary amine
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-3900000000-71dd24a94747bc71cff4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-af87edaa1391f623356d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-0900000000-b7d3f91cccbccb6109a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pc0-8900000000-7900a388e2a9753b49c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-f0fe7fc3060819b5a0a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-5e8fda07dbb8dc499d1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ue9-4900000000-15fa8f5d7c70fff9299d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-409c0f325aa5ec0f6a9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-4b51ded7059986756833 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ldi-9200000000-1b920371adb5ce1a24fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-a7afb973c05f34cd28a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-4c001580e59aedfbd58c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ik9-7900000000-0fe6bf21e9c43f76d242 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034169 |
|---|
| FooDB ID | FDB012455 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 21108245 |
|---|
| ChEBI ID | 142267 |
|---|
| PubChem Compound ID | 6826 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|