| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:13:24 UTC |
|---|
| Update Date | 2016-11-09 01:09:43 UTC |
|---|
| Accession Number | CHEM006412 |
|---|
| Identification |
|---|
| Common Name | 3-L-MENTHOXYPROPANE-1,2-DIOL |
|---|
| Class | Small Molecule |
|---|
| Description | 3-[[5-Methyl-2-(1-methylethyl)cyclohexyl]oxy]-1,2-propanediol is a physiological cooling agent used in food and beverage |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
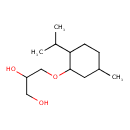
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (Menthyl)oxypropanediol | HMDB | | 3-((L-Menthyl)oxy)propane-1,2-diol | HMDB | | 3-L-(P-Menthane-3-yloxy)-1,2-propanediol | HMDB | | 3-L-Menthoxypropane-1,2-diol | HMDB, MeSH | | 3-Menthyloxy-1,2-propanediol | HMDB | | L-1,3-Menthoxypropane-1,2-diol | HMDB | | Menthoxypropanediol | HMDB | | Brachymelic primordial dwarfism | MeSH, HMDB | | Microcephalic osteodysplastic primordial dwarfism, type I | MeSH, HMDB | | MopdI | MeSH, HMDB | | Osteodysplastic primordial dwarfism, type I | MeSH, HMDB | | 3-Menthoxypropane-1,2-diol | MeSH, HMDB | | Mopd 1 | MeSH, HMDB | | Mopd1 | MeSH, HMDB | | Taybi linder syndrome | MeSH, HMDB | | Taybi-linder syndrome | MeSH, HMDB | | Microcephalic osteodysplastic primordial dwarfism, type 1 | MeSH, HMDB | | Mopd | MeSH, HMDB | | Cephaloskeletal dysplasia | MeSH, HMDB | | Low-birth-weight dwarfism with skeletal dysplasia | MeSH, HMDB | | Mopd I | MeSH, HMDB | | Osteodysplastic primordial dwarfism, type 1 | MeSH, HMDB |
|
|---|
| Chemical Formula | C13H26O3 |
|---|
| Average Molecular Mass | 230.344 g/mol |
|---|
| Monoisotopic Mass | 230.188 g/mol |
|---|
| CAS Registry Number | 87061-04-9 |
|---|
| IUPAC Name | 3-{[5-methyl-2-(propan-2-yl)cyclohexyl]oxy}propane-1,2-diol |
|---|
| Traditional Name | menthoxypropanediol |
|---|
| SMILES | CC(C)C1CCC(C)CC1OCC(O)CO |
|---|
| InChI Identifier | InChI=1S/C13H26O3/c1-9(2)12-5-4-10(3)6-13(12)16-8-11(15)7-14/h9-15H,4-8H2,1-3H3 |
|---|
| InChI Key | MDVYIGJINBYKOM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Menthane monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-menthane monoterpenoid
- Monocyclic monoterpenoid
- Glycerolipid
- Glycerol ether
- Secondary alcohol
- 1,2-diol
- Ether
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03e9-9410000000-98dfbfe970c670ae9068 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0pi9-9423000000-721c23d46517c900aa58 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-1590000000-2fadc1706d969b1f1f94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06s9-5950000000-9b54b9eae57a7880d8c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ap0-9300000000-95c723635e5dab45c5c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1690000000-8743ab1623786ad4be83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1910000000-e5047c0a6685c22bba58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-2900000000-750bdade2186d0de6440 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q1-9870000000-77e27c31dee07b091b21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-9500000000-5cab84e664f995a8a04e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056s-9000000000-9af9d01094ba4af570e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0390000000-063529b4cb405a75b479 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-e3a3687bd1938e74168d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k9i-1900000000-d7e3e42a4a0df375082e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036133 |
|---|
| FooDB ID | FDB014982 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4515105 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5362595 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|