| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:13:20 UTC |
|---|
| Update Date | 2016-11-09 01:09:43 UTC |
|---|
| Accession Number | CHEM006407 |
|---|
| Identification |
|---|
| Common Name | MENTHONE 1,2-GLYCEROL KETAL |
|---|
| Class | Small Molecule |
|---|
| Description | Menthone 1,2-glyceryl ketal, also known as menthone glycerin acetal or menthyl glycerin acetal, is a member of the class of compounds known as menthane monoterpenoids. Menthane monoterpenoids are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. p-Menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. Menthone 1,2-glyceryl ketal is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). Menthone 1,2-glyceryl ketal is used as a food additive (EAFUS: Everything Added to Food in the United States). Menthone 1,2-glyceryl ketal is a flavour enhancer for chewing gum and other low moisture foods. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
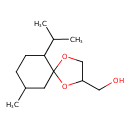
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hydroxymethyl-6-isopropyl-9-methyl-1,4-dioxaspiro[4.5]decane | HMDB | | 6-Isopropyl-9-methyl-1,4-dioxaspiro(4.5)decane-2-methanol | HMDB | | 9-Methyl-6-(1-methylethyl)-1,4-dioxaspiro[4.5]decane-2-methanol, 9ci | HMDB | | FEMA 3807 | HMDB | | FEMA 3808 | HMDB | | Frescolat | HMDB | | Menthone 1,2-glycerol ketal | HMDB | | Frescolat MGA | HMDB | | Menthone glycerin acetal | HMDB | | Menthyl glycerin acetal | HMDB | | Menthone glyceryl acetal | HMDB | | Menthone glyceryl ketal | HMDB | | MGA | HMDB | | L-Menthone 1,2-glycerol ketal | HMDB |
|
|---|
| Chemical Formula | C13H24O3 |
|---|
| Average Molecular Mass | 228.328 g/mol |
|---|
| Monoisotopic Mass | 228.173 g/mol |
|---|
| CAS Registry Number | 63187-91-7 |
|---|
| IUPAC Name | [9-methyl-6-(propan-2-yl)-1,4-dioxaspiro[4.5]decan-2-yl]methanol |
|---|
| Traditional Name | {6-isopropyl-9-methyl-1,4-dioxaspiro[4.5]decan-2-yl}methanol |
|---|
| SMILES | CC(C)C1CCC(C)CC11OCC(CO)O1 |
|---|
| InChI Identifier | InChI=1S/C13H24O3/c1-9(2)12-5-4-10(3)6-13(12)15-8-11(7-14)16-13/h9-12,14H,4-8H2,1-3H3 |
|---|
| InChI Key | ZBJCYZPANVLBRK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Menthane monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-menthane monoterpenoid
- Ketal
- Meta-dioxolane
- Oxacycle
- Organoheterocyclic compound
- Acetal
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9410000000-b382247dd270ed7bd196 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-06yc-9530000000-d3d1d3bd28f704c25a53 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1290000000-2a33c2ffa8194005ed41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9660000000-4dc87a745f4a98040054 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-9200000000-a353fa905f01dc6beef6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-2090000000-896a1d3ce21e9426591b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056s-7590000000-5f419ed783b43a5ae35f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f6x-9700000000-f21e3e951e7f6c37611a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-873da861442811ae04b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fb9-0790000000-051ca48c22c625175150 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ufs-1910000000-4da8dd1b3f946827b52c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-1e3bb0705c3f525a8fe0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9110000000-aa6fefeb9df2fe89aaf2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aou-9200000000-8c4be0723acde91d7cd4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036139 |
|---|
| FooDB ID | FDB014990 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 142428 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 162184 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|