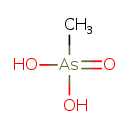| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:12:27 UTC |
|---|
| Update Date | 2016-11-09 01:09:42 UTC |
|---|
| Accession Number | CHEM006322 |
|---|
| Identification |
|---|
| Common Name | LINALYL HEXANOATE |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- IARC Carcinogens Group 2B
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| MAA | ChEBI | | MeAsO(OH)2 | ChEBI | | Methanearsonic acid | ChEBI | | Monomethylarsonic acid | ChEBI | | Methylarsonic acid | Kegg | | Methanearsonate | Generator | | Monomethylarsonate | Generator | | Dsma (jmaf) | HMDB | | Kyselina methylarsonova | HMDB | | Methyl arsonic acid | HMDB | | Methylarsenic acid | HMDB | | Methylarsinic acid | HMDB | | Monomethylarsinic acid | HMDB | | Disodium methanearsonate | MeSH, HMDB | | Monomethylarsonic acid, ammonium, iron (3+) salt | MeSH, HMDB | | Monomethylarsonic acid, dimercury (1+) salt | MeSH, HMDB | | Monomethylarsonic acid, disodium salt | MeSH, HMDB | | Monomethylarsonic acid, dipotassium salt | MeSH, HMDB | | Monomethylarsonic acid, iron (2+) salt (3:2) | MeSH, HMDB | | Monomethylarsonic acid, monoammonium salt | MeSH, HMDB | | Monomethylarsonic acid, monosodium salt | MeSH, HMDB | | Monomethylarsonic acid, zinc salt | MeSH, HMDB | | Methylarsonous acid | MeSH, HMDB | | Monomethylarsonic acid, iron salt | MeSH, HMDB | | Monomethylarsonic acid, monocalcium salt | MeSH, HMDB | | MSMA | MeSH, HMDB | | Monomethylarsonic acid, calcium salt (2:1) | MeSH, HMDB | | Monosodium methanearsonate | MeSH, HMDB | | Sodium methanearsonate | MeSH, HMDB |
|
|---|
| Chemical Formula | CH5AsO3 |
|---|
| Average Molecular Mass | 139.970 g/mol |
|---|
| Monoisotopic Mass | 139.945 g/mol |
|---|
| CAS Registry Number | 7779-23-9 |
|---|
| IUPAC Name | methylarsonic acid |
|---|
| Traditional Name | monomethylarsonic acid |
|---|
| SMILES | C[As](O)(O)=O |
|---|
| InChI Identifier | InChI=1S/CH5AsO3/c1-2(3,4)5/h1H3,(H2,3,4,5) |
|---|
| InChI Key | QYPPRTNMGCREIM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentaorganoarsanes. These are organoarsenic compounds containing an arsenic compound that is pentasubstituted by only organic groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organometallic compounds |
|---|
| Class | Organometalloid compounds |
|---|
| Sub Class | Organoarsenic compounds |
|---|
| Direct Parent | Pentaorganoarsanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentaorganoarsane
- Alkylarsine oxide
- Oxygen-containing organoarsenic compound
- Organic metalloid salt
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0900000000-da4d5da1a88bf0f506f4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-f551e617364ce9ae8012 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0900000000-2b6d3fe988a733ee8b82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0900000000-5b1e291e8a048e93b1bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dr-0900000000-faba21d1399d2be5f4cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-3900000000-c63ab6bc2dbdb52cf07f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-0900000000-af38e8044b6fdf1533a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fk9-0900000000-5b44146ed9e26a03f3f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0900000000-f2dd94a490343ba79810 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0900000000-59b264953374dcd1e76a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-387eed30d629c4fb0569 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-59b86cdf1af4cd9c1048 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dr-0900000000-2b854fae760c89475df5 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012258 |
|---|
| FooDB ID | FDB028897 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | METHYLARSONATE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Methylarsonic acid |
|---|
| Chemspider ID | 8604 |
|---|
| ChEBI ID | 29852 |
|---|
| PubChem Compound ID | 8948 |
|---|
| Kegg Compound ID | C07294 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|