| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:12:24 UTC |
|---|
| Update Date | 2016-11-09 01:09:42 UTC |
|---|
| Accession Number | CHEM006316 |
|---|
| Identification |
|---|
| Common Name | LINALOOL OXIDE PYRANOID |
|---|
| Class | Small Molecule |
|---|
| Description | Cnidiol C is found in citrus. Cnidiol C is a constituent of Citrus species.This is the pyranoid form of linalool oxide; there are 4 possible stereo-isomers |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
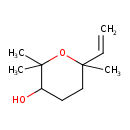
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,2,6-Trimethyl-6-vinyltetrahydro-2H-pyran-3-ol | HMDB | | 2,6,6-Trimethyl-2-vinyl-5-hydroxytetrahydropyran | HMDB | | 3-Hydroxy-2,2,6-trimethyl-6-vinyltetrahydropyran | HMDB | | 6-Ethenyl-3,4,5,6-tetrahydro-2,2,6-trimethyl-2H-pyran-3-ol | HMDB | | 6-Ethenyl-tetrahydro-2,2,6-trimethyl-2H-pyran-3-ol | HMDB | | 6-ethenyltetrahydro-2,2,6-Trimethyl-2H-pyran-3-ol | HMDB | | Linalool 3,7-oxide | HMDB | | Linalool oxide pyranoside | HMDB | | tetrahydro-2,2,6-Trimethyl-6-vinyl-2H-pyran-3-ol | HMDB |
|
|---|
| Chemical Formula | C10H18O2 |
|---|
| Average Molecular Mass | 170.249 g/mol |
|---|
| Monoisotopic Mass | 170.131 g/mol |
|---|
| CAS Registry Number | 14049-11-7 |
|---|
| IUPAC Name | 6-ethenyl-2,2,6-trimethyloxan-3-ol |
|---|
| Traditional Name | 6-ethenyl-2,2,6-trimethyloxan-3-ol |
|---|
| SMILES | CC1(C)OC(C)(CCC1O)C=C |
|---|
| InChI Identifier | InChI=1S/C10H18O2/c1-5-10(4)7-6-8(11)9(2,3)12-10/h5,8,11H,1,6-7H2,2-4H3 |
|---|
| InChI Key | BCTBAGTXFYWYMW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oxanes. Oxanes are compounds containing an oxane (tetrahydropyran) ring, which is a six-member saturated aliphatic heterocycle with one oxygen atom and five carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Oxanes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Oxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxane
- Secondary alcohol
- Oxacycle
- Ether
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9200000000-82ac429313d987d6c643 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00or-9820000000-9743b7c8b441a54d7e37 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-2900000000-7d503b1a5e93e182f71d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fzj-9400000000-aa02d627bea574941065 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ldr-9000000000-5be74a3b77b506049309 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1900000000-daff00d57437e851415c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-6900000000-ca6c09b40b7945d8ed84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-4e7a653f716f22157f7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00y0-9600000000-7b6fa02aa6435b837897 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9100000000-18150662edbd1892e3cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fr6-9000000000-d919d901703fa2e6b26e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-efbd93a1a83feefc94df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-7900000000-27d740975abbc5dd710f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-066r-9700000000-d018480f2e7db05adc2a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0035365 |
|---|
| FooDB ID | FDB014038 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00010325 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24591 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 26396 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|