| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:11:27 UTC |
|---|
| Update Date | 2016-11-09 01:09:42 UTC |
|---|
| Accession Number | CHEM006227 |
|---|
| Identification |
|---|
| Common Name | 2-ISOPROPYL-N,2,3-TRIMETHYLBUTYRAMIDE |
|---|
| Class | Small Molecule |
|---|
| Description | N,2,3-Trimethyl-2-(1-methylethyl)butanamide is a physiological stem cooling agent used in food; its effect is similar to that of menthol but without the strong minty flavour. N,2,3-Trimethyl-2-(1-methylethyl)butanamide is a flavouring agent for chewing gum and candie |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
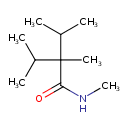
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Isopropyl-N,2,3-trimethylbutanamide | HMDB | | 2-Isopropyl-N,2,3-trimethylbutyramide | HMDB | | FEMA 3804 | HMDB | | Methyl diisopropyl propionamide | HMDB | | N,2,3-Trimethyl-2-(1-methylethyl)-butanamide | HMDB | | N,2,3-Trimethyl-2-(1-methylethyl)butanamide, 9ci | HMDB | | N,2,3-Trimethyl-2-isopropylbutanamide | HMDB | | Trimethyl isopropyl butanamide | HMDB |
|
|---|
| Chemical Formula | C10H21NO |
|---|
| Average Molecular Mass | 171.280 g/mol |
|---|
| Monoisotopic Mass | 171.162 g/mol |
|---|
| CAS Registry Number | 51115-67-4 |
|---|
| IUPAC Name | N,2,3-trimethyl-2-(propan-2-yl)butanamide |
|---|
| Traditional Name | trimethyl isopropyl butanamide |
|---|
| SMILES | CNC(=O)C(C)(C(C)C)C(C)C |
|---|
| InChI Identifier | InChI=1S/C10H21NO/c1-7(2)10(5,8(3)4)9(12)11-6/h7-8H,1-6H3,(H,11,12) |
|---|
| InChI Key | RWAXQWRDVUOOGG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl amines. N-acyl amines are compounds containing a fatty acid moiety linked to an amine group through an ester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty amides |
|---|
| Direct Parent | N-acyl amines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-amine
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ox-9800000000-c5b4234c60978dfd9470 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-406ac618b7b4f86227a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ec-1900000000-f7f0f0eeb36185e94058 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-9700000000-dcc03aa20052dd26d497 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-9f6d12a281c8575463fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1900000000-aaaed34c69799a7451fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fv-9500000000-aa17d39bb16c54d03f00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-5900000000-8bab6223028e44baac2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-022c-9800000000-6ccd57277bdb6070d508 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01b9-9000000000-6f3c6b8832f85e602c21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-fb5835943e4df6e70f22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-7b6cb732e26938e78c83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9100000000-77edd9fb89f8bd318385 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036195 |
|---|
| FooDB ID | FDB015050 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 58789 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 65300 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|