| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:11:05 UTC |
|---|
| Update Date | 2016-11-09 01:09:41 UTC |
|---|
| Accession Number | CHEM006190 |
|---|
| Identification |
|---|
| Common Name | ALPHA-ISOMETHYLIONYL ACETATE |
|---|
| Class | Small Molecule |
|---|
| Description | An acetate ester resulting from the formal condensation of the carboxy group of acetic acid with the hydroxy group of 3-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-ol. It is used a flavoring agent. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
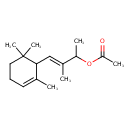
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3E)-3-Methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-yl acetate | ChEBI | | 1,2-Dimethyl-3-(2,6,6-trimethyl-2-cyclohexen-1-yl)propen-1-yl acetate | ChEBI | | 3-Methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-ol acetate | ChEBI | | 3-Methyl-4-(2,6,6-trimethylcyclohex-2-enyl)but-3-en-2-yl acetate | ChEBI | | FEMA 3845 | ChEBI | | Methyl alpha-ionylacetate | ChEBI | | Methyl-alpha-ionyl acetate | ChEBI | | (3E)-3-Methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-yl acetic acid | Generator | | 1,2-Dimethyl-3-(2,6,6-trimethyl-2-cyclohexen-1-yl)propen-1-yl acetic acid | Generator | | 3-Methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-ol acetic acid | Generator | | 3-Methyl-4-(2,6,6-trimethylcyclohex-2-enyl)but-3-en-2-yl acetic acid | Generator | | Methyl a-ionylacetate | Generator | | Methyl a-ionylacetic acid | Generator | | Methyl alpha-ionylacetic acid | Generator | | Methyl α-ionylacetate | Generator | | Methyl α-ionylacetic acid | Generator | | Methyl-a-ionyl acetate | Generator | | Methyl-a-ionyl acetic acid | Generator | | Methyl-alpha-ionyl acetic acid | Generator | | Methyl-α-ionyl acetate | Generator | | Methyl-α-ionyl acetic acid | Generator | | 3-Methyl-a-ionyl acetate | Generator | | 3-Methyl-a-ionyl acetic acid | Generator | | 3-Methyl-alpha-ionyl acetic acid | Generator | | 3-Methyl-α-ionyl acetate | Generator | | 3-Methyl-α-ionyl acetic acid | Generator | | a-Isomethylionyl acetate | HMDB | | alpha-Isomethylionyl acetate | HMDB | | (3E)-3-Methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-yl acetic acid | Generator |
|
|---|
| Chemical Formula | C16H26O2 |
|---|
| Average Molecular Mass | 250.376 g/mol |
|---|
| Monoisotopic Mass | 250.193 g/mol |
|---|
| CAS Registry Number | 68555-61-3 |
|---|
| IUPAC Name | (3E)-3-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-yl acetate |
|---|
| Traditional Name | (3E)-3-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-yl acetate |
|---|
| SMILES | CC(OC(C)=O)C(\C)=C\C1C(C)=CCCC1(C)C |
|---|
| InChI Identifier | InChI=1S/C16H26O2/c1-11-8-7-9-16(5,6)15(11)10-12(2)13(3)18-14(4)17/h8,10,13,15H,7,9H2,1-6H3/b12-10+ |
|---|
| InChI Key | TYUPZTIJMKMYHL-ZRDIBKRKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclofarsesane sesquiterpenoid
- Megastigmane sesquiterpenoid
- Sesquiterpenoid
- Ionone derivative
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01p6-9820000000-0b48363f97fa39a3df0b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0690000000-52a8d9384e805728f065 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000l-3920000000-a3254e225133c0b0424f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fsi-9310000000-537d0fd25462bb225c05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1190000000-c5dff820b4785015fa6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-4490000000-9c3406c3cfe8966b5591 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06r6-6910000000-ecba050f516e81b14d3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f7c-1920000000-1b25528500ca04897633 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01bi-4900000000-797a7867013c8ef3a06d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9700000000-b1b5c398ee92e5511f06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-2290000000-a7931d2e64e65058b836 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9300000000-c05cadaa265b92adf002 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-9e843328c1bcec52a520 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037631 |
|---|
| FooDB ID | FDB016747 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4942023 |
|---|
| ChEBI ID | 138772 |
|---|
| PubChem Compound ID | 6437467 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|