| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:09:37 UTC |
|---|
| Update Date | 2016-11-09 01:09:40 UTC |
|---|
| Accession Number | CHEM006065 |
|---|
| Identification |
|---|
| Common Name | HYDROXYLATED LECITHIN |
|---|
| Class | Small Molecule |
|---|
| Description | A non-proteinogenic L-alpha-amino acid that is L-asparagine hydroxylated at N-4. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
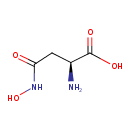
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| beta-Aspartohydroxamic acid | ChEBI | | beta-L-Aspartylhydroxamate | ChEBI | | L-Asparaginsaeure-4-hydroxyamid | ChEBI | | L-Aspartic acid beta-hydroxamate | ChEBI | | b-Aspartohydroxamate | Generator | | b-Aspartohydroxamic acid | Generator | | beta-Aspartohydroxamate | Generator | | Β-aspartohydroxamate | Generator | | Β-aspartohydroxamic acid | Generator | | b-L-Aspartylhydroxamate | Generator | | b-L-Aspartylhydroxamic acid | Generator | | beta-L-Aspartylhydroxamic acid | Generator | | Β-L-aspartylhydroxamate | Generator | | Β-L-aspartylhydroxamic acid | Generator | | L-Aspartate b-hydroxamate | Generator | | L-Aspartate beta-hydroxamate | Generator | | L-Aspartate β-hydroxamate | Generator | | L-Aspartic acid b-hydroxamic acid | Generator | | L-Aspartic acid beta-hydroxamic acid | Generator | | L-Aspartic acid β-hydroxamic acid | Generator | | 2-Amino-4-(hydroxyamino)-4-oxobutanoic acid | HMDB | | Aspartate-beta-hydroxamate | HMDB | | Aspartic acid beta-hydroxamate | HMDB | | beta-Aspartylhydroxamate | HMDB | | beta-Aspartylhydroxamic acid | HMDB | | D-Aspartic acid beta-hydroxamate | HMDB | | DL-Aspartic acid beta-hydroxamate monohydrate | HMDB | | L-Aspartylhydroxamate | HMDB | | N-Hydroxy-DL-asparagine | HMDB | | beta-Aspartylhydroxamic acid, (D)-isomer | MeSH | | beta-Aspartylhydroxamic acid, (L)-isomer | MeSH |
|
|---|
| Chemical Formula | C4H8N2O4 |
|---|
| Average Molecular Mass | 148.117 g/mol |
|---|
| Monoisotopic Mass | 148.048 g/mol |
|---|
| CAS Registry Number | 8029-76-3 |
|---|
| IUPAC Name | (2S)-2-amino-3-(hydroxycarbamoyl)propanoic acid |
|---|
| Traditional Name | L-aspartic acid β-hydroxamate |
|---|
| SMILES | N[C@@H](CC(=O)NO)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H8N2O4/c5-2(4(8)9)1-3(7)6-10/h2,10H,1,5H2,(H,6,7)(H,8,9)/t2-/m0/s1 |
|---|
| InChI Key | ZBYVTTSIVDYQSO-REOHCLBHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Fatty acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9200000000-c2dd8c82b8b9b0300f7b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0udi-6900000000-4dbb0a52851eb3b5b0da | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uea-2900000000-772fdfe0f8422a644e18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fki-9400000000-3a81aa62fa98f17d169e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-14e6157ef2e016194cae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-3900000000-6b1bd714783ba163f1ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ab9-9300000000-e9a84f870a19991c9831 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abc-9000000000-d30ec0fe1c08683b75dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004j-2900000000-27e75427da3b97fba34a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9300000000-9058e7769e8801b7e359 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-ca86a48626137a260d05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0g4i-6900000000-aed753e94d8f8f144645 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9100000000-ce2bf589a8bb9a6bfb81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-c7cab6e71d062a7ab811 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032332 |
|---|
| FooDB ID | FDB009602 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Hydroxylated lecithin |
|---|
| Chemspider ID | 88149 |
|---|
| ChEBI ID | 52794 |
|---|
| PubChem Compound ID | 44237312 |
|---|
| Kegg Compound ID | C03124 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|