| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:09:32 UTC |
|---|
| Update Date | 2016-11-09 01:09:40 UTC |
|---|
| Accession Number | CHEM006058 |
|---|
| Identification |
|---|
| Common Name | 4-HYDROXY-2,5-DIMETHYL-3(2H)-FURANONE |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of furans that is 2,5-dimethylfuran carrying additional oxo and hydroxy groups at positions 3 and 4 respectively. It has been found particularly in strawberries and other such fruits. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
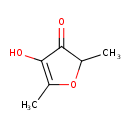
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,5-Dimethyl-3-hydroxy-4-oxo-4,5-dihydrofuran | ChEBI | | 2,5-Dimethyl-4-hydroxy-2,3-dihydrofuran-3-one | ChEBI | | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | ChEBI | | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | ChEBI | | 4-Hydroxy-2,5-dimethyl-furan-3(2H)-one | ChEBI | | Dimethylhydroxy furanone | ChEBI | | Furaneol | ChEBI | | HDMF | ChEBI | | Pineapple ketone | ChEBI | | Furaneol, (R)-isomer | MeSH, HMDB | | 4-HDMF | MeSH, HMDB | | Alletone | MeSH, HMDB | | Furaneol, (S)-isomer | MeSH, HMDB |
|
|---|
| Chemical Formula | C6H8O3 |
|---|
| Average Molecular Mass | 128.126 g/mol |
|---|
| Monoisotopic Mass | 128.047 g/mol |
|---|
| CAS Registry Number | 3658-77-3 |
|---|
| IUPAC Name | 4-hydroxy-2,5-dimethyl-2,3-dihydrofuran-3-one |
|---|
| Traditional Name | furaneol |
|---|
| SMILES | CC1OC(C)=C(O)C1=O |
|---|
| InChI Identifier | InChI=1S/C6H8O3/c1-3-5(7)6(8)4(2)9-3/h3,8H,1-2H3 |
|---|
| InChI Key | INAXVXBDKKUCGI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as furanones. Furanones are compounds containing a furan ring bearing a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dihydrofurans |
|---|
| Sub Class | Furanones |
|---|
| Direct Parent | Furanones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-furanone
- Vinylogous ester
- Cyclic ketone
- Ketone
- Oxacycle
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9100000000-8c4cb8aa573b16fcfb70 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0adu-9600000000-2c6fe7e0e944a33c5cc9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1900000000-2a171214e5cd4706888f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-3900000000-31e666e61cc7b8411876 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-054p-9000000000-af02647aeeb779640073 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-357e93488390a2976c9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-1900000000-602820cb95c6f5aecaae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9100000000-4e8ccb8034b60b5362d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-2900000000-f04fe270624eef9c155b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-9000000000-996faea12712159d1b18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-0c6cf150442af99bad61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-7900000000-afb6007af0c955aff56b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0avl-9000000000-61679df963f1143460b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mp-9000000000-67eb08c0013c727e892e | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040594 |
|---|
| FooDB ID | FDB020380 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00052712 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-10198 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Furaneol |
|---|
| Chemspider ID | 18218 |
|---|
| ChEBI ID | 76247 |
|---|
| PubChem Compound ID | 19309 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01694 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|