| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:08:00 UTC |
|---|
| Update Date | 2016-11-09 01:09:38 UTC |
|---|
| Accession Number | CHEM005919 |
|---|
| Identification |
|---|
| Common Name | N-(HEPTAN-4-YL)BENZO[D][1,3]DIOXOLE-5-CARBOXAMIDE |
|---|
| Class | Small Molecule |
|---|
| Description | N-(Heptan-4-yl)benzo[d][1,3]dioxole-5-carboxamide is used as a food additive [EAFUS] ("EAFUS: Everything Added to Food in the United States. [http://www.eafus.com/]") |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
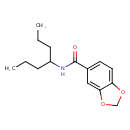
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-(Prop-2-en-1-yl)-1,3-benzodioxole-5-carboxamide | HMDB | | N-Allyl-1,3-benzodioxole-5-carboxamide | HMDB | | N-Allyl-3,4-(methylenedioxy)-benzamide | HMDB | | N-Allyl-3,4-(methylenedioxy)benzamide | HMDB | | N-(Heptan-4-yl)-2H-1,3-benzodioxole-5-carboximidate | HMDB |
|
|---|
| Chemical Formula | C15H21NO3 |
|---|
| Average Molecular Mass | 263.332 g/mol |
|---|
| Monoisotopic Mass | 263.152 g/mol |
|---|
| CAS Registry Number | 745047-51-2 |
|---|
| IUPAC Name | N-(heptan-4-yl)-2H-1,3-benzodioxole-5-carboxamide |
|---|
| Traditional Name | N-(heptan-4-yl)-2H-1,3-benzodioxole-5-carboxamide |
|---|
| SMILES | CCCC(CCC)NC(=O)C1=CC=C2OCOC2=C1 |
|---|
| InChI Identifier | InChI=1S/C15H21NO3/c1-3-5-12(6-4-2)16-15(17)11-7-8-13-14(9-11)19-10-18-13/h7-9,12H,3-6,10H2,1-2H3,(H,16,17) |
|---|
| InChI Key | YOBNUUGTIXQSPD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzodioxoles. These are organic compounds containing a benzene ring fused to either isomers of dioxole. Dioxole is a five-membered unsaturated ring of two oxygen atoms and three carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzodioxoles |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzodioxoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzodioxole
- Benzenoid
- Secondary carboxylic acid amide
- Carboxamide group
- Oxacycle
- Carboxylic acid derivative
- Acetal
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-6940000000-4c5e5fba854de5dd818c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0290000000-4bbd32ea3cf6839685c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-6690000000-09188a9a230d04354589 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006y-9400000000-cb2e7056fba1ca0a9302 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0190000000-603233881d4be9d4e75f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0490000000-f64655af46d49b2dd100 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01vp-9600000000-f1002636141fa8791475 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-b9021f5353998a04f5a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0090000000-6a8452df7c580f23a056 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9830000000-402ebb2fdbc9847c304a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0290000000-639f378c8798442546bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0690000000-d7be88aa632dec231dde | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-2900000000-2506919175ee89b1fba9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032305 |
|---|
| FooDB ID | FDB009549 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 17207458 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 22831877 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|