| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:07:49 UTC |
|---|
| Update Date | 2016-11-09 01:09:38 UTC |
|---|
| Accession Number | CHEM005906 |
|---|
| Identification |
|---|
| Common Name | 2,4-HEPTADIENAL |
|---|
| Class | Small Molecule |
|---|
| Description | A heptadienal in which the two double bonds are located at positions 2 and 4 (the E,E-geoisomer). |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
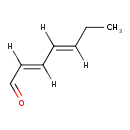
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,4E)-2,4-Heptadienal | ChEBI | | (e,e)-2,4-Heptadien-1-al | ChEBI | | (e,e)-2,4-Heptadienal | ChEBI | | 2,4-Heptadienal | ChEBI | | trans,trans-2,4-Heptadienal | ChEBI | | trans,trans-Hepta-2,4-dienal | ChEBI | | (2E,4Z)-2,4-Heptadienal | MeSH |
|
|---|
| Chemical Formula | C7H10O |
|---|
| Average Molecular Mass | 110.156 g/mol |
|---|
| Monoisotopic Mass | 110.073 g/mol |
|---|
| CAS Registry Number | 4313-03-5 |
|---|
| IUPAC Name | (2E,4E)-hepta-2,4-dienal |
|---|
| Traditional Name | 2,4-heptadienal, (E,E)- |
|---|
| SMILES | [H]\C(CC)=C(\[H])/C(/[H])=C(\[H])C=O |
|---|
| InChI Identifier | InChI=1S/C7H10O/c1-2-3-4-5-6-7-8/h3-7H,2H2,1H3/b4-3+,6-5+ |
|---|
| InChI Key | SATICYYAWWYRAM-VNKDHWASSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain aldehydes. These are an aldehyde with a chain length containing between 6 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Medium-chain aldehydes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain aldehyde
- Enal
- Alpha,beta-unsaturated aldehyde
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-6900000000-afce3b942bade13de496 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-9300000000-2bdf4b7bb26b9873d30a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1003-9000000000-a65f0c80d29149a34795 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1900000000-b4d0715e814862f94d02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-4900000000-c1c48aaeb5aa1897df8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-1cacd8acb7c46b17c8db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00lu-9000000000-f0bd4b794a66abf8efdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gbl-9000000000-ec55e0c5040565c60bd8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v09-9000000000-8a7e1086f1c5f4870361 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-4900000000-aff98f20a766bae26fb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-ec13dbe086b632466e3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-41569b3a125e752c5282 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0303844 |
|---|
| FooDB ID | FDB093546 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00053838 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4446442 |
|---|
| ChEBI ID | 132837 |
|---|
| PubChem Compound ID | 5283321 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|