| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:06:50 UTC |
|---|
| Update Date | 2016-11-09 01:09:37 UTC |
|---|
| Accession Number | CHEM005816 |
|---|
| Identification |
|---|
| Common Name | GERANIC ACID |
|---|
| Class | Small Molecule |
|---|
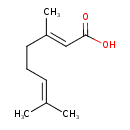
| Description | A polyunsaturated fatty acid that is octa-2,6-dienoic acid bearing two methyl substituents at positions 3 and 7 (the 2E-isomer). |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e)-3,7-Dimethylocta-2,6-dienoic acid | ChEBI | | 3,7-Dimethyl-2E,6-octadienoic acid | ChEBI | | 3,7-Dimethylocta-2,6-dienoate | ChEBI | | Geranilyc acid | ChEBI | | Geranoic acid | ChEBI | | trans-3,7-Dimethyl-2,6-octadien-1-Oic acid | ChEBI | | trans-Geranic acid | ChEBI | | trans-Geranoic acid | ChEBI | | Geranate | Kegg | | (e)-3,7-Dimethylocta-2,6-dienoate | Generator | | 3,7-Dimethyl-2E,6-octadienoate | Generator | | 3,7-Dimethylocta-2,6-dienoic acid | Generator | | Geranoate | Generator | | trans-3,7-Dimethyl-2,6-octadien-1-Oate | Generator | | trans-Geranate | Generator | | trans-Geranoate | Generator | | Decaprenoic acid | MeSH | | Decaprenoic acid, (Z)-isomer | MeSH | | Decaprenoic acid, (e)-isomer | MeSH |
|
|---|
| Chemical Formula | C10H16O2 |
|---|
| Average Molecular Mass | 168.236 g/mol |
|---|
| Monoisotopic Mass | 168.115 g/mol |
|---|
| CAS Registry Number | 459-80-3 |
|---|
| IUPAC Name | (2E)-3,7-dimethylocta-2,6-dienoic acid |
|---|
| Traditional Name | geranic acid |
|---|
| SMILES | CC(C)=CCC\C(C)=C\C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H16O2/c1-8(2)5-4-6-9(3)7-10(11)12/h5,7H,4,6H2,1-3H3,(H,11,12)/b9-7+ |
|---|
| InChI Key | ZHYZQXUYZJNEHD-VQHVLOKHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyclic monoterpenoids. These are monoterpenes that do not contain a cycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Acyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyclic monoterpenoid
- Medium-chain fatty acid
- Branched fatty acid
- Methyl-branched fatty acid
- Unsaturated fatty acid
- Fatty acyl
- Fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-066u-9300000000-18d0f7d4b2aac478b4d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ldi-0900000000-7d1a2fae1ccadeaa24ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-3900000000-102ac8789f85b97577f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ldl-9100000000-81898e9d56f862752c03 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-0900000000-b84dbb23a2126b008a00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xr-0900000000-1023ee5cb40f071209eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-6900000000-aea9515ceabda1985289 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0169534 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-7618 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Geranic_acid |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 67264 |
|---|
| PubChem Compound ID | 5275520 |
|---|
| Kegg Compound ID | C16461 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB005385 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|