| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:06:38 UTC |
|---|
| Update Date | 2016-11-09 01:09:36 UTC |
|---|
| Accession Number | CHEM005794 |
|---|
| Identification |
|---|
| Common Name | 3-(2-FUROYLTHIO)-2,5-DIMETHYLFURAN |
|---|
| Class | Small Molecule |
|---|
| Description | S-(2,5-Dimethyl-3-furanyl) 2-furancarbothioate is a flavouring agent with hydrolysed vegetable-type aroma and flavour. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
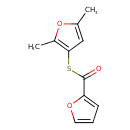
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| S-(2,5-Dimethyl-3-furanyl) 2-furancarbothioic acid | Generator | | 2,5-Dimethyl-3-(2-thiofuroyl)furan | HMDB | | 2,5-Dimethyl-3-thiofuroylfuran | HMDB | | 2-Furancarbothioic acid, S-(2,5-dimethyl-3-furanyl) ester | HMDB | | 3(2-furoylthio)-2,5-Dimethylfuran | HMDB | | 3-(2-furoylthio)-2,5-Dimethylfuran | HMDB | | S-(2,5-Dimethyl-3-furyl) thio-2-furoate | HMDB | | [(2,5-Dimethylfuran-3-yl)sulphanyl](furan-2-yl)methanone | Generator |
|
|---|
| Chemical Formula | C11H10O3S |
|---|
| Average Molecular Mass | 222.260 g/mol |
|---|
| Monoisotopic Mass | 222.035 g/mol |
|---|
| CAS Registry Number | 55764-31-3 |
|---|
| IUPAC Name | [(2,5-dimethylfuran-3-yl)sulfanyl](furan-2-yl)methanone |
|---|
| Traditional Name | [(2,5-dimethylfuran-3-yl)sulfanyl](furan-2-yl)methanone |
|---|
| SMILES | CC1=CC(SC(=O)C2=CC=CO2)=C(C)O1 |
|---|
| InChI Identifier | InChI=1S/C11H10O3S/c1-7-6-10(8(2)14-7)15-11(12)9-4-3-5-13-9/h3-6H,1-2H3 |
|---|
| InChI Key | IHJDHYVSIRCWIT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as furoic acid and derivatives. These are aromatic heterocyclic compounds containing a furan ring, which carries a carboxyl group or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Furans |
|---|
| Sub Class | Furoic acid and derivatives |
|---|
| Direct Parent | Furoic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Furoic acid or derivatives
- Aryl thioether
- Heteroaromatic compound
- Carbothioic s-ester
- Thiocarboxylic acid ester
- Oxacycle
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9310000000-3f38a97f7bea2d4f033d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00fr-1690000000-2a1d79e465193537a803 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-5790000000-94e353e342dc278bac4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9300000000-5558a425a68ff28e0030 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0490000000-293c2455ab11327fca2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0g4l-8960000000-9cb253bb696b5efa7c97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9300000000-cdcf0e0a7f805e3a96ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004l-9820000000-78c47cb2e4a2b177a69d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9300000000-d33e4db67f67e0c1eb64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-9300000000-ac14216cb86683326aa2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9000000000-325c53b5cb878354ab49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-a44c4c02db9e4b972019 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fvv-9000000000-bdc82088cafb245178d5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039585 |
|---|
| FooDB ID | FDB019210 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55942 |
|---|
| ChEBI ID | 166537 |
|---|
| PubChem Compound ID | 62106 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|