| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:03:04 UTC |
|---|
| Update Date | 2016-11-09 01:09:33 UTC |
|---|
| Accession Number | CHEM005508 |
|---|
| Identification |
|---|
| Common Name | DOG GRASS, EXTRACT (AGROPYRON REPENS (L.) BEAUV.) |
|---|
| Class | Small Molecule |
|---|
| Description | Digoxigenin is a cardenolide which is the aglycon of digoxin. Can be obtained by hydrolysis of digoxin or from Digitalis orientalis L. and Digitalis lanata Ehrh. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
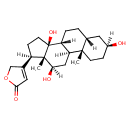
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Lanadigenin | MeSH |
|
|---|
| Chemical Formula | C23H34O5 |
|---|
| Average Molecular Mass | 390.513 g/mol |
|---|
| Monoisotopic Mass | 390.241 g/mol |
|---|
| CAS Registry Number | 977038-73-5 |
|---|
| IUPAC Name | 4-[(1S,2S,5S,7R,10R,11S,14R,15S,16R)-5,11,16-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]-2,5-dihydrofuran-2-one |
|---|
| Traditional Name | digoxigenin |
|---|
| SMILES | [H][C@@]1(CC[C@]2(O)[C@]3([H])CC[C@]4([H])C[C@@]([H])(O)CC[C@]4(C)[C@@]3([H])C[C@@]([H])(O)[C@]12C)C1=CC(=O)OC1 |
|---|
| InChI Identifier | InChI=1S/C23H34O5/c1-21-7-5-15(24)10-14(21)3-4-17-18(21)11-19(25)22(2)16(6-8-23(17,22)27)13-9-20(26)28-12-13/h9,14-19,24-25,27H,3-8,10-12H2,1-2H3/t14-,15+,16-,17-,18+,19-,21+,22+,23+/m1/s1 |
|---|
| InChI Key | SHIBSTMRCDJXLN-KCZCNTNESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cardenolides and derivatives. These are steroid lactones containing a furan-2-one moiety linked to the C17 atom of a cyclopenta[a]phenanthrene derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid lactones |
|---|
| Direct Parent | Cardenolides and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cardanolide-skeleton
- 3-hydroxysteroid
- 3-beta-hydroxysteroid
- 12-hydroxysteroid
- 14-hydroxysteroid
- Hydroxysteroid
- 2-furanone
- Cyclic alcohol
- Dihydrofuran
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Tertiary alcohol
- Carboxylic acid ester
- Secondary alcohol
- Lactone
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0btj-3931000000-dbf3a06930e85afcd289 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-004i-0009000000-306d8e9878a4c08a3e6b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0a4i-0109000000-d029e288691f96cf1f52 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-05fr-0009000000-0bec63b86c9e78fb334f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-01ox-0129000000-e2a7f0f6ad129a0727aa | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-0002-0019000000-f8a8b596c19812693065 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0btj-3931000000-dbf3a06930e85afcd289 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0009000000-c5f475cff1866a7f3219 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0c00-0019000000-2cbc7d7898be65963dbf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0r03-6927000000-53de5dd53f11c5d720e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-dd55347624d67cd11afe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ds-0009000000-a39e19800f4f97d48844 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-1109000000-f4b4c3ef514b487a8eb3 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB03671 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Digoxigenin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 42098 |
|---|
| PubChem Compound ID | 15478 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|