| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:02:31 UTC |
|---|
| Update Date | 2016-11-09 01:09:32 UTC |
|---|
| Accession Number | CHEM005464 |
|---|
| Identification |
|---|
| Common Name | 2,5-DIMETHYL-3-THIOISOVALERYLFURAN |
|---|
| Class | Small Molecule |
|---|
| Description | S-2,5-Dimethyl-3-furanyl 3-methylbutanethioate is a flavouring agent. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
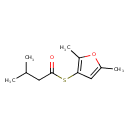
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| S-2,5-Dimethyl-3-furanyl 3-methylbutanethioic acid | Generator | | 2,5-Dimethyl-3-(thioisovaleryl)furan | HMDB | | 2,5-Dimethyl-3-thioisovaleryl furan | HMDB | | 2,5-Dimethyl-3-thioisovalerylfuran | HMDB | | 3-(isovalerylthio)-2,5-Dimethylfuran | HMDB | | FEMA 3482 | HMDB | | Isovaleric acid, thio-, S-2,5-dimethyl-3-furyl ester | HMDB | | S-(2,5-Dimethyl-3-furanyl) 3-methylbutanethioate | HMDB | | S-(2,5-Dimethyl-3-furyl) 3-methylbutanethioate | HMDB | | S-(2,5-Dimethyl-3-furyl) thioisovalerate | HMDB | | 1-[(2,5-Dimethylfuran-3-yl)sulphanyl]-3-methylbutan-1-one | Generator |
|
|---|
| Chemical Formula | C11H16O2S |
|---|
| Average Molecular Mass | 212.309 g/mol |
|---|
| Monoisotopic Mass | 212.087 g/mol |
|---|
| CAS Registry Number | 55764-28-8 |
|---|
| IUPAC Name | 1-[(2,5-dimethylfuran-3-yl)sulfanyl]-3-methylbutan-1-one |
|---|
| Traditional Name | 1-[(2,5-dimethylfuran-3-yl)sulfanyl]-3-methylbutan-1-one |
|---|
| SMILES | CC(C)CC(=O)SC1=C(C)OC(C)=C1 |
|---|
| InChI Identifier | InChI=1S/C11H16O2S/c1-7(2)5-11(12)14-10-6-8(3)13-9(10)4/h6-7H,5H2,1-4H3 |
|---|
| InChI Key | XFNLWIPNTYNNJX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aryl thioethers. These are organosulfur compounds containing a thioether group that is substituted by an aryl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Thioethers |
|---|
| Sub Class | Aryl thioethers |
|---|
| Direct Parent | Aryl thioethers |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aryl thioether
- Fatty acyl thioester
- Furan
- Heteroaromatic compound
- Carbothioic s-ester
- Thiocarboxylic acid ester
- Oxacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004l-9600000000-b3f84a5405fc9b270e05 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-2940000000-4869c3cf256e32248c77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-9720000000-70ecc300abd53f2e4792 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9200000000-1f378596a55910a0ed51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01t9-4940000000-f37e1464aa83ceb37145 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-057i-9600000000-32b4ff437fc962a3ddc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-015c-9100000000-d0e096f1f835b6bbb4f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-7910000000-c706a975f5dd90b54511 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004r-8900000000-656eeefca05c8f132ec7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00mp-9200000000-71ea3bae5ae440c0b8ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0910000000-d1c536f27ef5492dbc8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-8900000000-6ce6cea27bf4fb789776 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9100000000-305eec6480d28af69861 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037153 |
|---|
| FooDB ID | FDB016148 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 37932 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 41570 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|