| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:02:31 UTC |
|---|
| Update Date | 2016-11-09 01:09:32 UTC |
|---|
| Accession Number | CHEM005463 |
|---|
| Identification |
|---|
| Common Name | 2-(2-BUTYL)-4,5-DIMETHYL-3-THIAZOLINE |
|---|
| Class | Small Molecule |
|---|
| Description | 2,5-Dihydro-4,5-dimethyl-2-(1-methylpropyl)thiazole is a flavouring ingredient. 2,5-Dihydro-4,5-dimethyl-2-(1-methylpropyl)thiazole is reported in hydrolysed vegetable protein. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
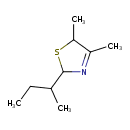
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(2-Butyl)-4,5-dimethyl-3-thiazoline | HMDB | | FEMA 3619 | HMDB |
|
|---|
| Chemical Formula | C9H17NS |
|---|
| Average Molecular Mass | 171.303 g/mol |
|---|
| Monoisotopic Mass | 171.108 g/mol |
|---|
| CAS Registry Number | 65894-82-8 |
|---|
| IUPAC Name | 2-(butan-2-yl)-4,5-dimethyl-2,5-dihydro-1,3-thiazole |
|---|
| Traditional Name | 4,5-dimethyl-2-(sec-butyl)-2,5-dihydro-1,3-thiazole |
|---|
| SMILES | CCC(C)C1SC(C)C(C)=N1 |
|---|
| InChI Identifier | InChI=1S/C9H17NS/c1-5-6(2)9-10-7(3)8(4)11-9/h6,8-9H,5H2,1-4H3 |
|---|
| InChI Key | FLBOQJFNAYJWIA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiazolines. These are heterocyclic compounds containing a five-member unsaturated aliphatic ring with one nitrogen atom, one sulfur atom, three carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azolines |
|---|
| Sub Class | Thiazolines |
|---|
| Direct Parent | Thiazolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Meta-thiazoline
- Ketimine
- Azacycle
- Dialkylthioether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Thioether
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Imine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9500000000-0c462b95972d73e843c4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-3900000000-456705494053ee8f9649 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-3900000000-2380194c284da976721d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-9000000000-27d8e93f69394bff5ead | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0229-1900000000-903957013642889ab6b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00e9-8900000000-33bf662aec92f6facbd1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uei-9200000000-1da683d60ae90e25976b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-2432cc3097e2e34a7647 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-2900000000-d669e0ac5e14bf7e5d8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9100000000-5ea82e9c15688b779541 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-eb36bce95656a659f1d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kmi-5900000000-267b81c0f19bcbcde32f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-7900000000-61ea07bbb66df8bc8a2e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037514 |
|---|
| FooDB ID | FDB016593 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4515079 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5362564 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|