| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:02:12 UTC |
|---|
| Update Date | 2016-11-09 01:09:32 UTC |
|---|
| Accession Number | CHEM005440 |
|---|
| Identification |
|---|
| Common Name | N-3,7-DIMETHYL-2,6-OCTADIENYLCYCLOPROPYLCARBOXAMIDE |
|---|
| Class | Small Molecule |
|---|
| Description | An N-(3,7-dimethylocta-2,6-dien-1-yl)cyclopropanecarboxamide in which the double bond at position 2 adopts an E-configuration. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
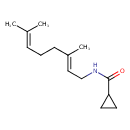
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E)-N-3,7-Dimethyl-2,6-octadienylcyclopropylcarboxamide | ChEBI | | (e)-N-3,7-Dimethyl-2,6-octadienylcyclopropylcarboxamide | ChEBI | | FEMA 4267 (e form) | ChEBI | | N-[(2E)-3,7-Dimethyl-2,6-octadien-1-yl]cyclopropanecarboxamide | ChEBI | | N-[(2E)-3,7-Dimethyl-2,6-octadienyl]cyclopropanecarboxamide | ChEBI | | N-[(2Z)-3,7-Dimethylocta-2,6-dienyl]cyclopropanecarboxamide | HMDB | | N-[(2E)-3,7-Dimethylocta-2,6-dien-1-yl]cyclopropanecarboximidate | Generator |
|
|---|
| Chemical Formula | C14H23NO |
|---|
| Average Molecular Mass | 221.339 g/mol |
|---|
| Monoisotopic Mass | 221.178 g/mol |
|---|
| CAS Registry Number | 744251-93-2 |
|---|
| IUPAC Name | N-[(2E)-3,7-dimethylocta-2,6-dien-1-yl]cyclopropanecarboxamide |
|---|
| Traditional Name | N-[(2E)-3,7-dimethylocta-2,6-dien-1-yl]cyclopropanecarboxamide |
|---|
| SMILES | CC(C)=CCC\C(C)=C\CNC(=O)C1CC1 |
|---|
| InChI Identifier | InChI=1S/C14H23NO/c1-11(2)5-4-6-12(3)9-10-15-14(16)13-7-8-13/h5,9,13H,4,6-8,10H2,1-3H3,(H,15,16)/b12-9+ |
|---|
| InChI Key | UKNMSFRSBQONET-FMIVXFBMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monocyclic monoterpenoids. These are monoterpenoids containing 1 ring in the isoprene chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Monocyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocyclic monoterpenoid
- Cyclopropanecarboxylic acid or derivatives
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014l-9300000000-8b6134db22152ed4fb17 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-4890000000-9a9b0f09a14a5bc124b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-9610000000-2cc88c42b780bc084c4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9100000000-d4d872c9b12b5df72875 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1190000000-8fcef029e1f9b2c8c37b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9770000000-89b8b1256ed3ed4aed15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-34925186fd61da40e7d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0190000000-035fbe2146db96e545d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-9540000000-e8a520e7dfabc8a6c809 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-66697a9f4ecf9f1dd5cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01bc-9120000000-ef2f76d44af2e27f8446 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-9000000000-e7a66179b3922de5d61b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-115570d33c8ea120bab7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032240 |
|---|
| FooDB ID | FDB009327 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9833352 |
|---|
| ChEBI ID | 173636 |
|---|
| PubChem Compound ID | 11658617 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|