| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:02:06 UTC |
|---|
| Update Date | 2016-11-09 01:09:32 UTC |
|---|
| Accession Number | CHEM005431 |
|---|
| Identification |
|---|
| Common Name | 2,5-DIMETHYL-4-METHOXY-3(2H)-FURANONE |
|---|
| Class | Small Molecule |
|---|
| Description | Mesifurane is found in fruits. Mesifurane is a constituent of various fruits including raspberry, strawberry, mango, pineapple, blackberry and cape gooseberry. Mesifurane is a flavouring agent |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
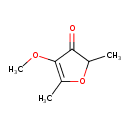
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,5-Dimethyl 4-methoxy furan-3-one | HMDB | | 2,5-Dimethyl-4-methoxy-2,3-dihydro-3-furanone | HMDB | | 2,5-Dimethyl-4-methoxy-3(2H)-furanone | HMDB, MeSH | | 3(2H)-Furanone, 2,5-dimethyl-4-methoxy | HMDB | | 4-Methoxy-2,5-dimethyl-2,3-dihydrofuran-3-one | HMDB | | 4-Methoxy-2,5-dimethyl-3(2H)-furanone | HMDB, MeSH | | 4-Methoxy-2,5-dimethylfuran-3(2H)-one | HMDB | | FEMA 3664 | HMDB | | Mesifuran | HMDB | | O-Methylfuraneol | HMDB | | DMMF CPD | MeSH, HMDB |
|
|---|
| Chemical Formula | C7H10O3 |
|---|
| Average Molecular Mass | 142.153 g/mol |
|---|
| Monoisotopic Mass | 142.063 g/mol |
|---|
| CAS Registry Number | 4077-47-8 |
|---|
| IUPAC Name | 4-methoxy-2,5-dimethyl-2,3-dihydrofuran-3-one |
|---|
| Traditional Name | 4-methoxy-2,5-dimethyl-2H-furan-3-one |
|---|
| SMILES | COC1=C(C)OC(C)C1=O |
|---|
| InChI Identifier | InChI=1S/C7H10O3/c1-4-6(8)7(9-3)5(2)10-4/h4H,1-3H3 |
|---|
| InChI Key | SIMKGHMLPVDSJE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as furanones. Furanones are compounds containing a furan ring bearing a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dihydrofurans |
|---|
| Sub Class | Furanones |
|---|
| Direct Parent | Furanones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-furanone
- Vinylogous ester
- Cyclic ketone
- Ketone
- Oxacycle
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00xs-9100000000-70c7d96673888cf7153e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-96041d3e7b9e509669d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-3900000000-f393b47f20abc98d8752 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05xv-9100000000-1530efdfde178d0c52ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1900000000-63bd61312fa748005768 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-597d2aedc6ce435c8b4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gb9-9100000000-e762db83470b78aba435 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-85d0a280c7f5a1bd1cda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-ef4b1ee7a42d0ff86a01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00l6-9000000000-cda6f5debcbdf45c92ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9600000000-88c12d56e1e21abfb121 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-9200000000-ae47c9fb31c272e95e6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-8f6293982824844fbbf1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034230 |
|---|
| FooDB ID | FDB012541 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 55261 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61325 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|