| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:01:57 UTC |
|---|
| Update Date | 2016-11-09 01:09:32 UTC |
|---|
| Accession Number | CHEM005418 |
|---|
| Identification |
|---|
| Common Name | 2,6-DIMETHYL-3-((2-METHYL-3-FURYL)THIO)-4-HEPTANONE |
|---|
| Class | Small Molecule |
|---|
| Description | 2,6-Dimethyl-3-[(2-methyl-3-furanyl)thio]-4-heptanone is a flavouring ingredient. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
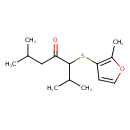
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,3-Diisopropylacetonyl 2-methyl-3-furyl sulfide | HMDB | | 2,6-Dimethyl-3-((2-methyl-3-furanyl)thio)-4-heptanone | HMDB | | 2,6-Dimethyl-3-((2-methyl-3-furyl)thio)-4-heptanone | HMDB | | 2,6-Dimethyl-3-((2-methyl-3-furyl)thio)heptan-4-one | HMDB | | 3-((2-Methyl-3-furyl)thio)-2,6-dimethyl-4-heptanone | HMDB | | FEMA 3538 | HMDB | | 2,6-Dimethyl-3-[(2-methylfuran-3-yl)sulphanyl]heptan-4-one | Generator |
|
|---|
| Chemical Formula | C14H22O2S |
|---|
| Average Molecular Mass | 254.388 g/mol |
|---|
| Monoisotopic Mass | 254.134 g/mol |
|---|
| CAS Registry Number | 61295-51-0 |
|---|
| IUPAC Name | 2,6-dimethyl-3-[(2-methylfuran-3-yl)sulfanyl]heptan-4-one |
|---|
| Traditional Name | 2,6-dimethyl-3-[(2-methylfuran-3-yl)sulfanyl]heptan-4-one |
|---|
| SMILES | CC(C)CC(=O)C(SC1=C(C)OC=C1)C(C)C |
|---|
| InChI Identifier | InChI=1S/C14H22O2S/c1-9(2)8-12(15)14(10(3)4)17-13-6-7-16-11(13)5/h6-7,9-10,14H,8H2,1-5H3 |
|---|
| InChI Key | PFFLSEMCPNTWOY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aryl thioethers. These are organosulfur compounds containing a thioether group that is substituted by an aryl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Thioethers |
|---|
| Sub Class | Aryl thioethers |
|---|
| Direct Parent | Aryl thioethers |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aryl thioether
- Alkylarylthioether
- Heteroaromatic compound
- Furan
- Ketone
- Oxacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08g3-9610000000-49c369d7a021008a8839 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0bt9-1790000000-a39919a0003865ad3a5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0btc-9450000000-cef2f97fb0d7e1ae90fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p9-9500000000-bb1fadb4ebd57e3dd8fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0w29-1590000000-25f8e482a9469969e341 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-5920000000-092566cb2f4708a6a52c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9300000000-4cdf03466d6980a49860 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-6900000000-ff7a5c59270217874f58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-6900000000-a2b3bb35a7701dffa8e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01p9-9400000000-e3c82e2a55c213de762e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-2910000000-883e39aad08f70b34480 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-8900000000-621b19bce756ca80e12a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9800000000-9e256309b7d3e4059137 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037154 |
|---|
| FooDB ID | FDB016149 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 56003 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 62183 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|