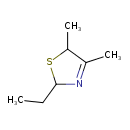| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:01:53 UTC |
|---|
| Update Date | 2016-11-09 01:09:32 UTC |
|---|
| Accession Number | CHEM005413 |
|---|
| Identification |
|---|
| Common Name | 4,5-DIMETHYL-2-ETHYL-3-THIAZOLINE |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Ethyl-2,5-dihydro-4,5-dimethylthiazole is maillard produced in foods. 2-Ethyl-2,5-dihydro-4,5-dimethylthiazole is a flavouring agent for meats and soups, doubtless as a mixture of cis- and trans-forms. |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,5-dihydro-4,5-Dimethyl-2-ethyl-thiazole | HMDB | | 2-Ethyl-2,5-dihydro-4,5-dimethyl-thiazole | HMDB | | 2-Ethyl-4,5-dimethyl thiazoline | HMDB | | 2-Ethyl-4,5-dimethyl-3-thiazoline | HMDB | | 4,5-Dimethyl-2-ethyl-3-thiazoline | HMDB | | FEMA 3620 | HMDB |
|
|---|
| Chemical Formula | C7H13NS |
|---|
| Average Molecular Mass | 143.250 g/mol |
|---|
| Monoisotopic Mass | 143.077 g/mol |
|---|
| CAS Registry Number | 76788-46-0 |
|---|
| IUPAC Name | 2-ethyl-4,5-dimethyl-2,5-dihydro-1,3-thiazole |
|---|
| Traditional Name | 2-ethyl-4,5-dimethyl-2,5-dihydro-1,3-thiazole |
|---|
| SMILES | CCC1SC(C)C(C)=N1 |
|---|
| InChI Identifier | InChI=1S/C7H13NS/c1-4-7-8-5(2)6(3)9-7/h6-7H,4H2,1-3H3 |
|---|
| InChI Key | CSACPVARWHDAET-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiazolines. These are heterocyclic compounds containing a five-member unsaturated aliphatic ring with one nitrogen atom, one sulfur atom, three carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azolines |
|---|
| Sub Class | Thiazolines |
|---|
| Direct Parent | Thiazolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Meta-thiazoline
- Ketimine
- Azacycle
- Dialkylthioether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Thioether
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Imine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06vi-9300000000-4e3c9044eb1f067714ad | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1900000000-23430be94c5f3ac1c66c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-3900000000-23895cd5912b8ae2b8cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kbf-9000000000-7ec84d4a1376c4801af5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0016-8900000000-baafed9028f577bcf06e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000x-9500000000-7fa2403998511ee4f2de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0089-9000000000-863e3c336277cf999b61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-4900000000-d1d9813b60db9f646661 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9100000000-2178f540a47f59416412 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-8900000000-ee73000d25bbc60ab576 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-0e5bfc080e096b750c97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6x-4900000000-ec81c597887c374b364a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-bdb66fe54ade4d081770 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037176 |
|---|
| FooDB ID | FDB016172 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4515096 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5362584 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|