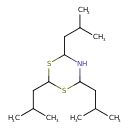| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 02:01:10 UTC |
|---|
| Update Date | 2016-11-09 01:09:31 UTC |
|---|
| Accession Number | CHEM005362 |
|---|
| Identification |
|---|
| Common Name | DIHYDRO-2,4,6-TRIS(2-METHYLPROPYL)-4H-1,3,5-DITHIAZINE |
|---|
| Class | Small Molecule |
|---|
| Description | Dihydro-2,4,6-tris(2-methylpropyl)-4h-1,3,5-dithiazine is used as a food additive [EAFUS] ("EAFUS: Everything Added to Food in the United States. [http://www.eafus.com/]") |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,3,5-Dithiazine, perhydro-2,4-dimethyl-6-(1-methylethyl) | HMDB | | 5,6-Dihydro-2,4-dimethyl-6-isopropyl-4H-1,3,5-dithiazine | HMDB |
|
|---|
| Chemical Formula | C15H31NS2 |
|---|
| Average Molecular Mass | 289.543 g/mol |
|---|
| Monoisotopic Mass | 289.190 g/mol |
|---|
| CAS Registry Number | 74595-94-1 |
|---|
| IUPAC Name | 2,4,6-tris(2-methylpropyl)-1,3,5-dithiazinane |
|---|
| Traditional Name | 2,4,6-tris(2-methylpropyl)-1,3,5-dithiazinane |
|---|
| SMILES | CC(C)CC1NC(CC(C)C)SC(CC(C)C)S1 |
|---|
| InChI Identifier | InChI=1S/C15H31NS2/c1-10(2)7-13-16-14(8-11(3)4)18-15(17-13)9-12(5)6/h10-16H,7-9H2,1-6H3 |
|---|
| InChI Key | RQGPQWUKHADVPF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3,5-dithiazinanes. These are cyclic compounds that contain a dithiazinane ring, which is a saturated heterocycle that consisting of one nitrogen atom, two sulfur atoms at the 1-,3-, and 5- position, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azacyclic compounds |
|---|
| Sub Class | Dithiazinanes |
|---|
| Direct Parent | 1,3,5-dithiazinanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3,5-dithiazinane
- Thioacetal
- Dialkylthioether
- Hemithioaminal
- Thioether
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001l-4490000000-435cf8594ef4baea4524 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1290000000-8b41f79d76d27daf8925 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fdo-2590000000-c7b47ab975dc9cfeb23d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1000-9400000000-6b3a450cb7d0609e9bcb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f79-1930000000-45c4c89b8f7a8efd71ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-3900000000-ed277bdd1c7f1f08ac12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-5900000000-b41ec12a4ac700b72347 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-691aea92afedb6995644 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-1390000000-068e4604c053c1c5e120 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxr-9720000000-0c0ec71aae6b5034908d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-c6ca9e7660a09b36a766 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-523b6184e8d6f0b34aaa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00yr-3690000000-8f723b53e53de231c342 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032221 |
|---|
| FooDB ID | FDB009292 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 21106002 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 46941523 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|